Detail description of Lithophyllum okamurae (Lithophylloideae, Corallinales), a widely distributed crustose coralline alga in marine ecosystems
-
Abstract: Lithophyllum okamurae is one of the important encrusting coralline algae, which plays important roles as primary producer, carbonate sediment builder, and habitat provider in the marine ecosystems. In this study, L. okamurae was collected from tropical coast of Sanya, and firstly described based on both detailed morph-anatomical characteristics and molecular studies of typic DNA sequences. The structure of the thalli of L. okamurae was pseudoparenchymatous construction with radially organized dimerous organizations in the crustose portion. The pseudoparenchymatous construction were composed of three parts, including 1 to 3 layers of epithelia cells which had flatten to round outermost walls, one layer of square or rectangular cells of the hypothallia and multiple layers of square or elongated rectangular peripheral cells. Palisade cells were observed, and the cells of the contiguous vegetative filaments were connected by secondary pit-connections with cell fusions absent. The carposporangial conceptacles, the spermatangial conceptacles, the bisporangial conceptacles and the tetrasporangial conceptacles were observed, and all these four kinds of conceptacles were uniporate. The spermatangial conceptacles were slightly convex and buried at shallow depths in the thalli tissues, and the carposporangial conceptacles and asexual conceptacles were protruding and conical. Phylogenetic studies based on DNA barcoding markers of 18S rDNA, COI, rbcL and psbA revealed that L. okamurae clustered with the closest relation of L. atlanticum, and formed a distinct branch. Based on the comparative anatomical features and the molecular data, the detailed description of the valid species of L. okamurae was firstly given in this study to provide theoretical basis for algae resources utilization and conservation in marine ecosystems.
-
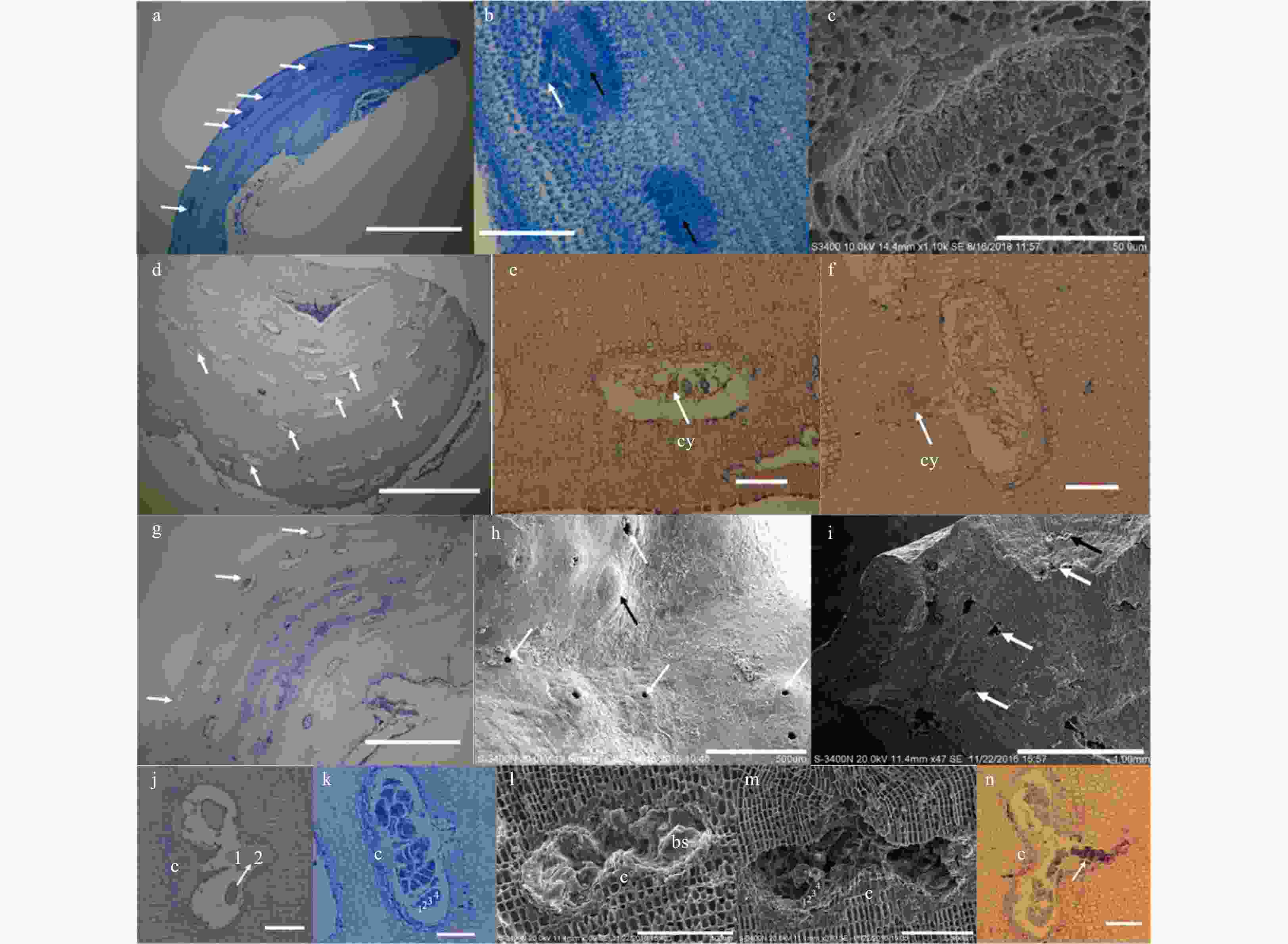
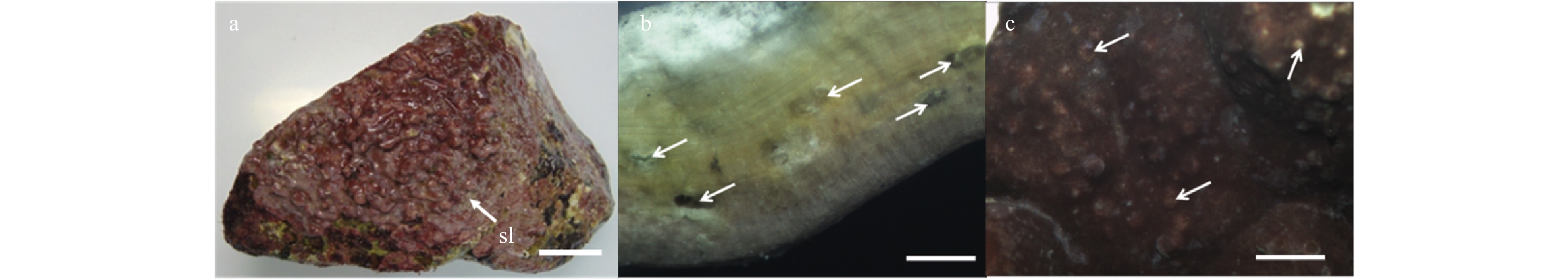
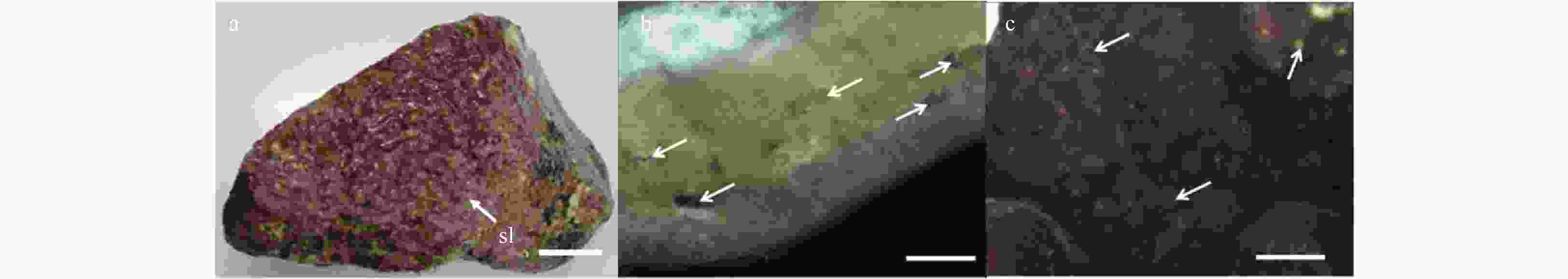
Figure 1. Vegetative structures of Lithophyllum okamurae. a. External morphology of alga individuals growing on rocks, and sloughing cells (sl) in sheets form residual on the crust (white arrow). Scale bar is 3 cm. b. Cross-section showing the pseudoparenchymatous construction of the alga thallus, and conceptacles (white arrow) in it. Scale bar is 5 mm. c. Surface view of the thallus, showing conceptacles (white arrow) distributed on both surface and branches. Scale bar is 5 mm.
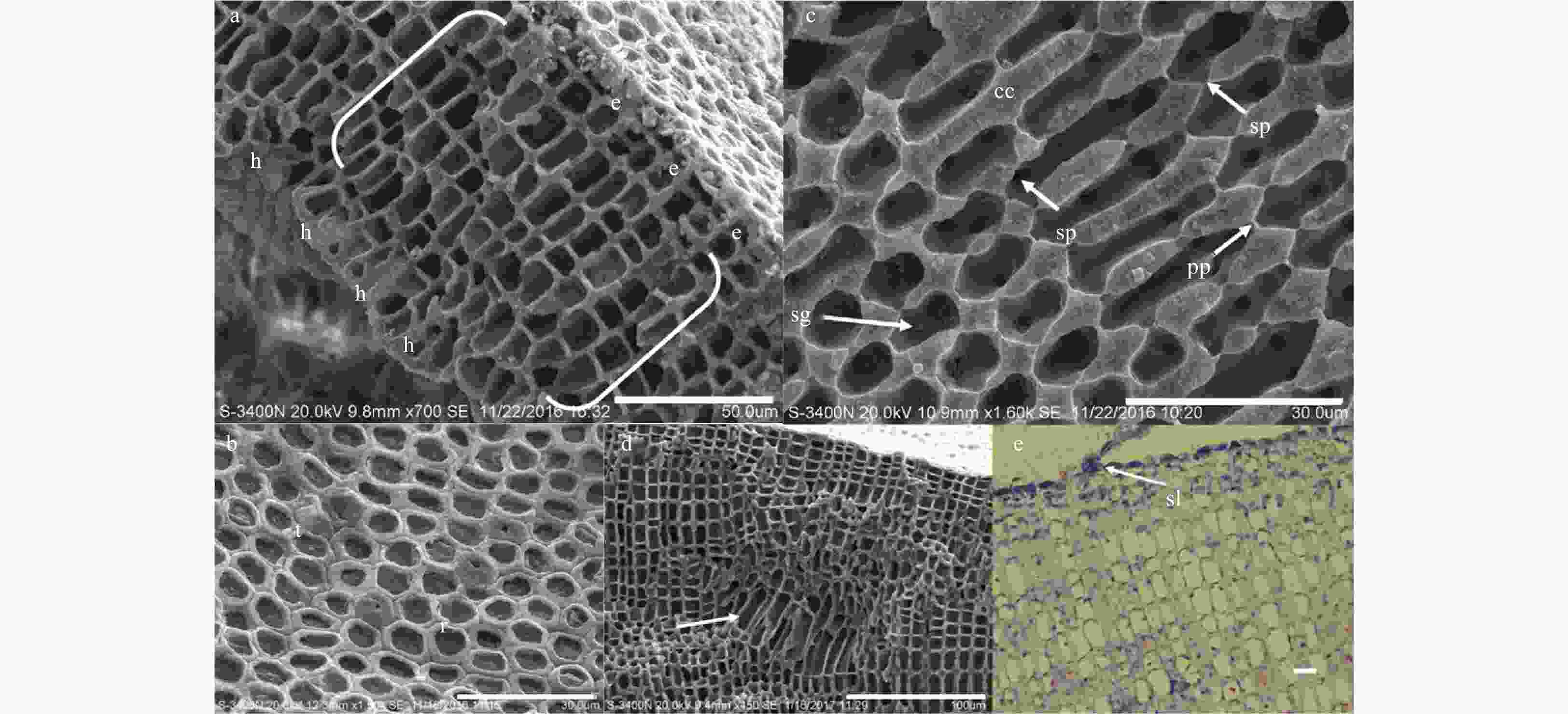
Figure 2. Anatomical features of Lithophyllum okamurae. a. Vertical fracture showing the thallus crust with dimerous organization, and unistratose hypothallium (h), perithallium (bracket) and epithallium (e) visible. Scale bar is 50 μm. b. Surface view of polygonal epithallial cells with intact (t) and lost (r) roofs. The outer peripheral parts of epithallial cells with lost roofs were uncalcified. Scale bar is 30 μm. c. Vertical fracture showing primary pit connections (pp) and secondary pit connections (sp) among the filaments in peripheral region of the crusts, and calcium carbonate (cc) deposited in the cell walls, and floridean starch grains (sg) stored in the perithallium cells. Scale bar is 30 μm. d. Palisade cells (white arrow) in perithallium part of the crust. Scale bar is 100 μm. e. Epithallium cells sloughing (sl) in form of face peeling under optical microscope. Scale bar is 10 μm.
Figure 3. Anatomical features of fertile crusts and the conceptacles of Lithophyllum okamurae. a. Cross section through a male plant showing spermatangial conceptacles (white arrow) of different developing stages. Scale bar is 1 mm. b. Two spermatangial conceptacles showing apical oblique divisions forming spermatangia (white arrow), and the spermatangia were releasing from the pore (black arrow). Scale bar is 100 μm. c. A spermatangial conceptacle in elder production stage, where spermatangia were produced on the floor of the conceptacle chamber. Scale bar is 50 μm. d. Cross section through a female plant showing cystocarpic conceptacles (white arrow) of different developing stages. Scale bar is 1 mm. e. A developing cystocarpic conceptacle with several cystocarps (cy) in the chamber. Scale bar is 50 μm. f. A cystocarpic conceptacle which was releasing its cystocarps (cy). Scale bar is 50 μm. g. Cross section through an asexual plant showing tetrasporangial or bisporangial conceptacles (white arrow). Scale bar is 1 mm. h. Surface view of a fertile thallus showing uniporate (white arrow) conceptacles, and a uniporate conceptacle with steepled topped roofs (black arrow). Scale bar is 500 μm. i. A fertile tetrasporophyte thalli with a bisporangial conceptacle (black arrow) and several tetrasporangial conceptacles (white arrow) originated in the crust. Scale bar is 1 mm. j. A bisporangial conceptacle with zonate bisporangia (white arrow) and central columella (c) in the chamber, with the bisporangia was releasing. Scale bar is 50 μm. k. A tetrasporangial conceptacle with zonate bisporangia (white arrow) and central columella (c) in the chamber. Scale bar is 50 μm. l. A bisporangial conceptacle with roof and showing putative zonately arranged bisporangia (bs), and the bisporangia arranged around a prominent central columella. Scale bar is 100 μm. m. A tetrasporangial conceptacle with roof showing putative zonately arranged tetrasporangia, and the tetrasporangia arranged around a prominent central columella (c). Scale bar is 100 μm. n. A tetrasporangial conceptacle which was releasing its tetrasporangia. Scale bar is 50 μm.
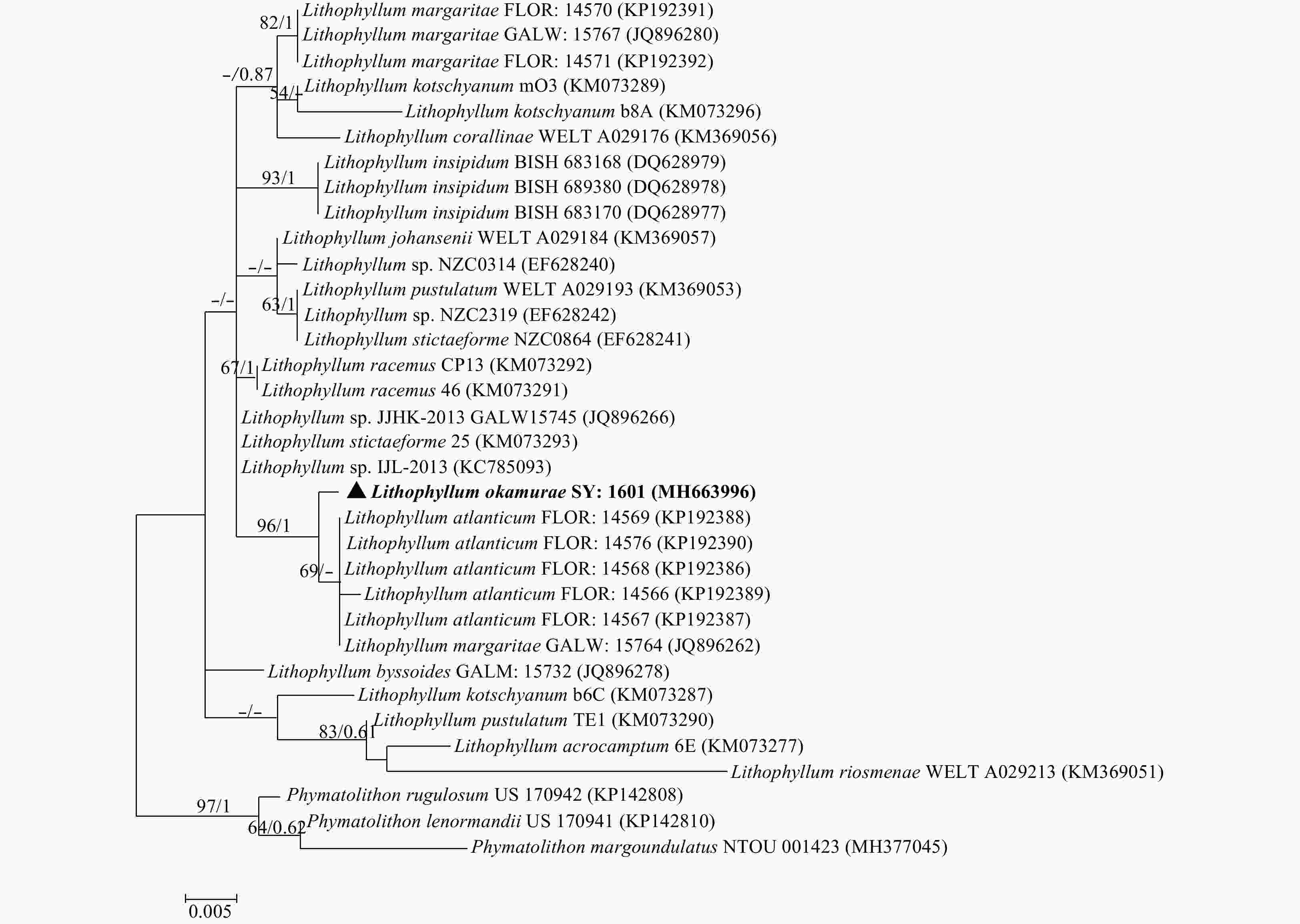
Figure 4. Tree constructed with ML for the 18S rDNA alignment. Values at branches represent distance analyses of 1 000 bootstrap replicates (left value) and Bayesian posterior probabilities (right value). Branches lacking values received <50% support. GenBank accession numbers provided. The newly generated sequence was shown in bold.
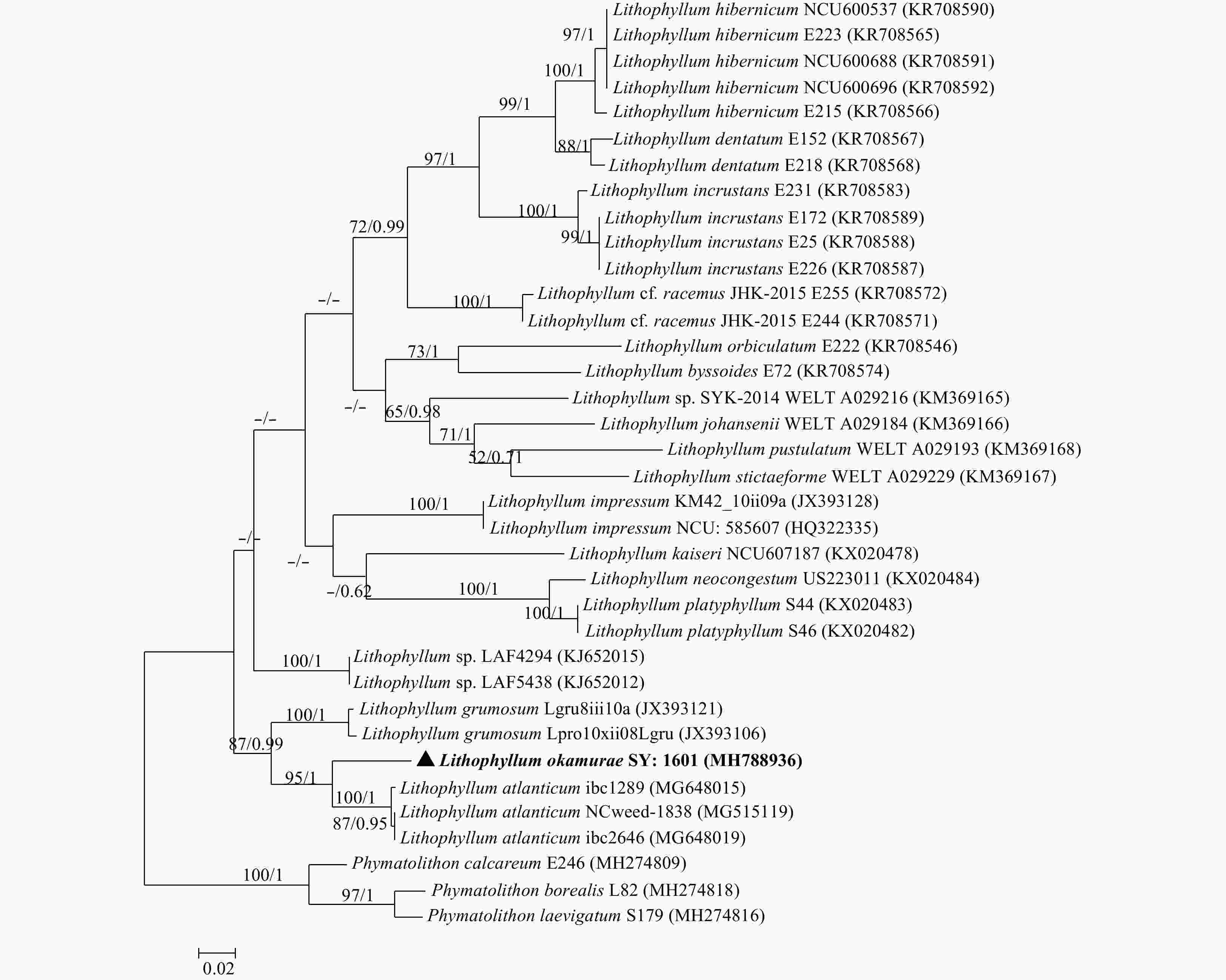
Figure 5. Tree constructed with ML for the rbcL alignment. Values at branches represent distance analyses of 1 000 bootstrap replicates (left value) and Bayesian posterior probabilities (right value). Branches lacking values received <50% support. GenBank accession numbers provided. The newly generated sequence was shown in bold.
Table 1. Four pairs of primers used to perform the sequences
Primer Sequence Reference 18S-G01 5′-CACCTGGTTGATCCTGCCAG-3′ Harper and Saunders (2001) 18S-G14 5′-CTTGGCAGACGCTTTCGCAG-3′ Harper and Saunders (2001) COI-F 5′-TCAACAAATCATAAAGATATTGG-3′ Saunders (2005) COI-R 5′-ACTTCTGGATGTCCAAAAAAYCA-3′ Saunders (2005) rbcL-090F 5′-CCATATGCYAAAATGGGATATTGG-3′ Yoon et al. (2002) R-rbcS start 5′-TGTGTTGCGGCCGCCCTTGTGTTAGTCTCAC-3′ Yoon et al. (2002) psbA-F 5′-ATGACTGCTACTTTAGAAAGACG-3′ Yoon et al. (2002) psbA-R2 5′-TCATGCATWACTTCCATACCTA-3′ Yoon et al. (2002) Table 2. Comparison of species in genus Lithophyllum that share characteristics with Lithophyllum okamurae (present study)
L.
okamurae
(present study)L.
atlanticum
(1)L.
margaritae
(1)L.
incrustans
(2)L.
incrassatum
(2)L.
neoatalayense
(2)L.
corallinae
(3)L.
kotschyanum
(3)L.
neofarlowii
(3)L.
pygmaeum
(3)L.
yessoense
(3)Substratum rocks, corals and shells free living and on rocks free living rocks and debris rocks rocks or spongites growing on Corallina spp. growing on corals rocks ND ND Epithallium Layer 1–3 1–2 1–4 1 ≥5 1 1 1 3–5 Diameter/μm 4.0–7.0/5.0–9.0 3.0–6.0 5.0–13.0 3.0–4.0 5.0–12.0 4.0–6.0 6.6–8.0 5.0–7.0 5.0–10.0 6.6–8.0 Length/μm 4.0–7.0/3.0–6.0 6.0–11.0 1.5–5.0 3.0–4.0 3.0–5.0 2.0–5.0 3.3–5.0 3.0–5.0 4.0–7.0 3.3–5.0 Hypothallium Layer 1 1 ND 2–3 1 Diameter/μm 7.0–13.0/4.0–7.0 5.5–11.0 3.0–10.0 12.0–15.0 9.0–22.0 6.0–15.0 6.9–9.9 7.0–10.0/5.0–10.0 5.0–6.0 5.0–7.0/5.0–10.0 6.6–9.9 Length/μm 7.0–13.0/13.0–17.0 8.0–17.0 7.0–16.0 9.0–11.0 9.0–22.0 3.0–10.0 19.8–23.1(–30.0) 7.0–10.0/7.0–20.0 10.0–13.2 5.0–7.0/7.0–23.0 5.0–6.6 Peripheral Diameter/μm 8.0–11.0/(5.0–)7.0–8.0 (–10.0) 4.5–10.0 4.0–8.0 3.0–35.0 6.0–12.0 9.0–11.0 6.6–9.9 5.0–13.0 3.3–5.0 8.0–16.0 (–23.0)/6.0–13.0 5.0–7.0 Length/μm 8.0–11.0/(10.0–) 12.0–17.0 (–23.0) 6.5–13.0 4.0–13.0 3.0–9.0 6.0–13.0 9.0–11.0 16.5–26.4 (13.0–)15.0–30.0 (–36.0) 6.6–9.9 35.0–60.0/7.0–22.0 5.0–8.0 Spermatangial Chamber diameter/μm n=15 (123.0–) 138.0–163.0 (–175.0) 156.0–269.0 130.0–221.0 72.6–89.1 125.4–224.5 Chamber height/μm (38.0–) 52.5–62.5 (–80.0) 33.0–52.0 39.0–73.0 42.9–52.8 33.0–46.2 Spermatangia Diameter/μm n=15 (2.6–)3.1–3.7(–4.1) Length/μm 10.5–12.5(–14.1) Carposporangial conceptacles Chamber diameter/μm n=20 (159.6–) 167.5–201.5 (–223.0) 132.0–148.5 Chamber height/μm 82.0–112.0 (–131.0) 66.0–108.9 Carposporangia Diameter n=15 19.3–26.0 23.1–29.7 Length 11.3–17.5 13.2–23.1 Asexual conceptacles Chamber diameter/μm n=20 (170.0–) 240.0–265.0 (–315.0) 315.0–345.0 180.0–220.0 260.0–350.0 208.0–364.0 229.0–252.0 168.3–198.0 270.0–360.0 125.4–165.0 220.0–300.0 138.6–141.9 Chamber height/μm (100.0–)125.0–150.0 90.0–130.0 70.0–95.0 110.0 52.0–130.0 83.0–177.0 99.0–105.6 79.2–105.6 75.0–130.0 66.0–112.2 Tetra/Bisporangia Diameter n=15 9.9–23.1(–28.5) 15.0–33.0 15.0–35.0 20.0–35.0 27.0–38.0 26.0–34.0 16.5–26.4 25.0–33.0 16.5–19.8 26.0–34.0 13.2–36.3 Length (39.0–)39.6–52.8(–73.2) 46.0–68.0 40.0–60.0 45.0–80.0 46.0–65.0 65.0–91.0 49.8–59.4 42.0–50.0 39.6–49.5 50.0–58.0 29.7–66.0 -
[1] Adey W H, Adey P J. 1973. Studies on the biosystematics and ecology of the epilithic crustose Corallinaceae of the British Isles. British Phycological Journal, 8(4): 343–407. doi: 10.1080/00071617300650381 [2] Adey W H, Hernandez-Kantun J J, Johnson G, et al. 2015. DNA sequencing, anatomy, and calcification patterns support a monophyletic, subarctic, carbonate reef-forming Clathromorphum (Hapalidiaceae, Corallinales, Rhodophyta). Journal of Phycology, 51(1): 189–203. doi: 10.1111/jpy.12266 [3] Basso D, Caragnano A, Le Gall L, et al. 2015. The genus Lithophyllum in the north-western Indian Ocean, with description of L. yemenense sp. nov., L. socotraense sp. nov., L. subplicatum comb. et stat. nov., and the resumed L. affine, L. kaiseri, and L. subreduncum (Rhodophyta, Corallinales). Phytotaxa, 208(3): 183–200. doi: 10.11646/phytotaxa.208.3.1 [4] Basso D, Caragnano A, Rodondi G. 2014. Trichocytes in Lithophyllum kotschyanum and Lithophyllum spp. (Corallinales, Rhodophyta) from the NW Indian Ocean. Journal of Phycology, 50(4): 711–717. doi: 10.1111/jpy.12197 [5] Basso D, Rodondi G. 2006. A Mediterranean population of Spongites fruticulosus (Rhodophyta, Corallinales), the type species of Spongites, and the taxonomic status of S. stalactitica and S. racemosa. Phycologia, 45(4): 403–416. doi: 10.2216/04-93.1 [6] Basso D, Rodondi G, Mari M. 2004. A comparative study between Lithothamnion minervae and the type material of Millepora fasciculata (Corallinales, Rhodophyta). Phycologia, 43(2): 215–223. doi: 10.2216/i0031-8884-43-2-215.1 [7] Braga J C, Aguirre J. 2004. Coralline algae indicate Pleistocene evolution from deep, open platform to outer barrier reef environments in the northern Great Barrier Reef margin. Coral Reefs, 23(4): 547–558 [8] Chamberlain Y M. 1996. Lithophylloid Corallinaceae (Rhodophyta) of the genera Lithophyllum and Titanoderma from southern Africa. Phycologia, 35(3): 204–221. doi: 10.2216/i0031-8884-35-3-204.1 [9] Chihara M. 1974. The significance of reproductive and spore germination characteristics to the systematic of the Corallinaceae: nonarticulated coralline algae. Journal of Phycology, 10(3): 266–274 [10] Clarkston B E, Saunders G W. 2010. A comparison of two DNA barcode markers for species discrimination in the red algal family Kallymeniaceae (Gigartinales, Florideophyceae), with a description of Euthora timburtonii sp. nov. Botany, 88(2): 119–131. doi: 10.1139/B09-101 [11] da Gama B A P, Plouguerné E, Pereira R C. 2014. The antifouling defence mechanisms of marine macroalgae. Advances in Botanical Research, 71: 413–440. doi: 10.1016/B978-0-12-408062-1.00014-7 [12] Ding Lanping, Huang Bingxin, Wang Hongwei. 2015. New classification system of marine red algae of China. Guangxi Sciences (in Chinese), 22(2): 164–188 [13] Drummond A J, Rambaut A, Shapiro B, et al. 2005. Bayesian coalescent inference of past population dynamics from molecular sequences. Molecular Biology and Evolution, 22(5): 1185–1192. doi: 10.1093/molbev/msi103 [14] Guiry M D, Guiry G M. 2018. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org [2016-08-27/2018-12-13] [15] Hajibabaei M, Singer G A C, Hebert P D N, et al. 2007. DNA barcoding: how it complements taxonomy, molecular phylogenetics and population genetics. Trends in Genetics, 23(4): 167–172. doi: 10.1016/j.tig.2007.02.001 [16] Hall T A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41: 95–98 [17] Harper J T, Saunders G W. 2001. The application of sequences of the ribosomal cistron to the systematics and classification of the Florideophyte red algae (Florideophyceae, Rhodophyta). Cahiers de Biologie Marine, 42(1): 25–38 [18] Harvey A S, Phillips L E, Woelkerling W J, et al. 2006. The Corallinaceae, subfamily Mastophoroideae (Corallinales, Rhodophyta) in south-eastern Australia. Australian Systematic Botany, 19(5): 387–429. doi: 10.1071/SB05029 [19] Harvey A, Woelkerling W J. 2007. A guide to nongeniculate coralline red algal (Corallinales, Rhodophyta) rhodolith identification. Ciencias Marinas, 33(4): 411–426. doi: 10.7773/cm.v33i4.1210 [20] Hernández-Kantún J J, Gabrielson P, Hughey J R, et al. 2016. Reassessment of branched Lithophyllum spp. (Corallinales, Rhodophyta) in the Caribbean Sea with global implications. Phycologia, 55(6): 619–639. doi: 10.2216/16-7.1 [21] Hernández-Kantún J J, Rindi F, Adey W H, et al. 2015. Sequencing type material resolves the identity and distribution of the generitype Lithophyllum incrustans, and related European species L. bathyporum (Corallinales, Rhodophyta). Journal of Phycology, 51(4): 791–807. doi: 10.1111/jpy.12319 [22] Johansen H W. 1976. Current status of generic concepts in coralline algae (Rhodophyta). Phycologia, 15(2): 221–244. doi: 10.2216/i0031-8884-15-2-221.1 [23] Keats D W, Knight M A, Pueschel C M. 1997. Antifouling effects of epithallial shedding in three crustose coralline algae (Rhodophyta, Coralinales) on a coral reef. Journal of Experimental Marine Biology and Ecology, 213(2): 281–293. doi: 10.1016/S0022-0981(96)02771-2 [24] Kundal P. 2011. Generic distinguishing characteristics and stratigraphic ranges of fossil corallines: an update. Journal of the Geological Society of India, 78(6): 571–586. doi: 10.1007/s12594-011-0119-z [25] Li Xiubao, Titlyanova T V, Titlyanov E A, et al. 2018. Coral Reef Marine Plants of Hainan Island (in Chinese). Beijing: Science Press, 1–242 [26] Liu L C, Lin S M, Caragnano A, et al. 2018. Species diversity and molecular phylogeny of non-geniculate coralline algae (Corallinophycidae, Rhodophyta) from Taoyuan algal reefs in northern Taiwan, including Crustaphytum gen. nov. and three new species. Journal of Applied Phycology, 30(6): 3455–3469. doi: 10.1007/s10811-018-1620-1 [27] Maggs C A, Verbruggen H, De Clerck O. 2007. Molecular systematics of red algae: building future structures on firm foundations. In: Brodie J, Lewis J, eds. Unravelling the Algae: The Past, Present, and Future of Algal Systematics. The Systematics Association Special Volume Series. Boca Raton, FL: CRC Press, 103–121 [28] Maneveldt G W, Chamberlain Y M, Keats D W. 2008. A catalogue with keys to the non-geniculate coralline algae (Corallinales, Rhodophyta) of South Africa. South African Journal of Botany, 74(4): 555–566. doi: 10.1016/j.sajb.2008.02.002 [29] McCoy S J, Kamenos N A. 2015. Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change. Journal of Phycology, 51(1): 6–24. doi: 10.1111/jpy.12262 [30] Nelson W A. 2009. Calcified macroalgae-critical to coastal ecosystems and vulnerable to change: a review. Marine and Freshwater Research, 60(8): 787–801. doi: 10.1071/MF08335 [31] Nelson W A, Sutherland J E, Farr T J, et al. 2015. Multi-gene phylogenetic analyses of New Zealand coralline algae: Corallinapetra novaezelandiae gen. et sp. nov. and recognition of the Hapalidiales ord. nov. Journal of Phycology, 51(3): 454–468. doi: 10.1111/jpy.12288 [32] Nylander J A A. 2004. MrModeltest v2. Program distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University [33] Nylund G M, Pavia H. 2005. Chemical versus mechanical inhibition of fouling in the red alga Dilsea carnosa. Marine Ecology Progress Series, 299: 111–121. doi: 10.3354/meps299111 [34] Peña V, Hernandez-Kantun J J, Adey W H, et al. 2018. Assessment of coralline species diversity in the European coasts supported by sequencing of type material: the case study of Lithophyllum nitorum (Corallinales, Rhodophyta). Cryptogamie, Algologie, 39(1): 123–137. doi: 10.7872/crya/v39.iss1.2018.123 [35] Pezzolesi L, Falace A, Kaleb S, et al. 2017. Genetic and morphological variation in an ecosystem engineer, Lithophyllum byssoides (Corallinales, Rhodophyta). Journal of Phycology, 53(1): 146–160. doi: 10.1111/jpy.12488 [36] Phang S M, Yeong H Y, Ganzon-Fortes E T, et al. 2016. Marine algae of the South China Sea bordered by Indonesia, Malaysia, Philippines, Singapore, Thailand and Vietnam. Raffles Bulletin of Zoology Supplement, 34: 13–59 [37] Pueschel C M, Keats D W. 1997. Fine structure of deep-layer sloughing and epithallial regeneration in Lithophyllum neoatalayense (Corallinales, Rhodophyta). Phycological Research, 45(1): 1–8. doi: 10.1111/j.1440-1835.1997.tb00056.x [38] Richards J L, Gabrielson P W, Fredericq S. 2014. New insights into the genus Lithophyllum (Lithophylloideae, Corallinaceae, Corallinales) from deepwater rhodolith beds offshore the NW Gulf of Mexico. Phytotaxa, 190(1): 162–175. doi: 10.11646/phytotaxa.190.1.11 [39] Richards J L, Gabrielson P W, Hughey J R, et al. 2018. A re-evaluation of subtidal Lithophyllum species (Corallinales, Rhodophyta) from North Carolina, USA, and the proposal of L. searlesii sp. nov. Phycologia, 57(3): 318–330. doi: 10.2216/17-110.1 [40] Riosmena-Rodríguez R, Nelson W, Aguirre J. 2017. Rhodolith/Maërl Beds: A Global Perspective. Cham: Springer, 2–362 [41] Riosmena-Rodríguez R, Woelkerling W J, Foster M S. 1999. Taxonomic reassessment of rhodolith-forming species of Lithophyllum (Corallinales, Rhodophyta) in the Gulf of California, Mexico. Phycologia, 38(5): 401–417. doi: 10.2216/i0031-8884-38-5-401.1 [42] Rösler A, Perfectti F, Peña V, et al. 2016. Phylogenetic relationships of Corallinaceae (Corallinales, Rhodophyta): taxonomic implications for reef-building corallines. Journal of Phycology, 52(3): 412–431. doi: 10.1111/jpy.12404 [43] Saunders G W. 2005. Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philosophical Transactions of the Royal Society B: Biological Sciences, 360(1462): 1879–1888. doi: 10.1098/rstb.2005.1719 [44] Steneck R S. 1986. The ecology of coralline algal crusts: convergent patterns and adaptative strategies. Annual Review of Ecology and Systematics, 17: 273–303. doi: 10.1146/annurev.es.17.110186.001421 [45] Tamura K, Peterson D, Peterson N, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10): 2731–2739. doi: 10.1093/molbev/msr121 [46] Torrano-Silva B N, Vieira B R, Riosmena-Rodríguez R, et al. 2018. Guidelines for DNA barcoding of coralline algae, focusing on Lithophylloideae (Corallinales) from Brazil. Botanica Marina, 61(2): 127–140. doi: 10.1515/bot-2017-0040 [47] van der Heijden L H, Kamenos N A. 2015. Reviews and syntheses: calculating the global contribution of coralline algae to total carbon burial. Biogeosciences, 12(21): 6429–6441. doi: 10.5194/bg-12-6429-2015 [48] Verlaque M. 2010. Field-methods to analyse the condition of Mediterranean Lithophyllum byssoides (Lamarck) Foslie rims. Scientific Reports of Port-Cros National Park, 24: 185–196 [49] Vidal R, Meneses I, Smith M. 2003. Molecular genetic identification of crustose representatives of the order Corallinales (Rhodophyta) in Chile. Molecular Phylogenetics and Evolution, 28(3): 404–419. doi: 10.1016/S1055-7903(03)00123-4 [50] Vieira-Pinto T, Oliveira M C, Bouzon J, et al. 2014. Lithophyllum species from Brazilian coast: range extension of Lithophyllum margaritae and description of Lithophyllum atlanticum sp. nov. (Corallinales, Corallinophycidae, Rhodophyta). Phytotaxa, 190(1): 355–369. doi: 10.11646/phytotaxa.190.1.21 [51] Villas-Boas A B, Riosmena-Rodríguez R, Amado-Filho G M, et al. 2009. Rhodolith-forming species of Lithophyllum (Corallinales; Rhodophyta) from Espírito Santo State, Brazil, including the description of L. depressum sp. nov. Phycologia, 48(4): 237–248. doi: 10.2216/08-35.1 [52] Woelkerling W J. 1983. A taxonomic reassessment of Lithothamnium (Corallinaceae, Rhodophyta) based on studies of R. A. Philippi’s original collections. British Phycological Journal, 18(2): 165–197. doi: 10.1080/00071618300650211 [53] Woelkerling W J. 1988. The Coralline Red Algae: An Analysis of the Genera and Subfamilies of Nongeniculate Corallinaceae. Oxford, New York: Oxford University Press, 1–268 [54] Woelkerling W J. 1996. Subfamily lithophylloideae. In: Womersley H B S, ed. The Marine Benthic Flora of Southern Australia. Part IIIB. Gracilariales, Rhodymeniales, Corallinales and Bonnemaisoniales. Canberra: Australian Biological Resources Study, 214–237 [55] Woelkerling W J, Campbell S J. 1992. An account of southern Australian species of Lithophyllum (Corallinaceae, Rhodophyta). Bulletin of the British Museum of (Natural History) Botany Series, 22(1): 1–107 [56] Woelkerling W J, Gustavsen G, Myklebost H E, et al. 2005. The coralline red algal herbarium of Mikael Foslie: revised catalogue with analyses. Gunneria, 77: 1–625 [57] Woelkerling W J, Irvine L M, Harvey A S. 1993. Growth-forms in non-geniculate coralline red algae (Corallinales, Rhodophyta). Australian Systematic Botany, 6(4): 277–293. doi: 10.1071/SB9930277 [58] Xia Bangmei. 2004. Flora Algarum Marinarum Sinicarum Tomus II Rhodophyta No. IV Corallinales (in Chinese). Beijing: Science Press, 1–147 [59] Yoon H S, Hackett J D, Bhattacharya D. 2002. A single origin of the peridinin- and fucoxanthin-containing plastids in dinoflagellates through tertiary endosymbiosis. Proceedings of the National Academy of Sciences of the United States of America, 99(18): 11724–11729. doi: 10.1073/pnas.172234799 -




 下载:
下载: