| Citation: | Jilong Wang, Wei Liu, Peilun Li, Fujiang Tang, Wanqiao Lu, Jian Yang, Tao Jiang. Evidence of return of chum salmon released from Tangwang River by strontium marking method[J]. Acta Oceanologica Sinica, 2021, 40(8): 182-186. doi: 10.1007/s13131-021-1792-4 |
Tangwang River is a cold-water river and a major downstream tributary of Songhua River, the largest tributary of the Amur River in the northeast of China. It inhabits many species of fish especially cold-water species, such as chum salmon (Oncorhynchus keta), Amur whitefish (Coregonus ussuriensis) and so on. Chum salmon belong to typical anadromous fish of which life history includes freshwater and marine life stages. Chum salmon were born in rivers, the juvenile fish will migrage to the sea soon after hatching and they live in the ocean until maturation and then go upstream to the river where they were born to reproduce. They die soon after breeding and reproduce only once in their life. At present, the habitat distribution of chum salmon in China has decreased significantly, and Tangwang River was once one of the important spawning rivers. Tangwang River once had several important spawning grounds of chum salmon before 1980s. However, the fishery resources have undergone serious depletion due to anthropogenic factors such as overfishing, habitat degradation and pollution (Liu, 2011; Yang et al., 2015). Especially, the chum salmon had not been seen for almost three decades. In order to restore the fish resources, enhancement release of chum salmon has been conducted since 2012. And it was found that a small number of chum salmon migrated to Tangwang River in autumn in recent years. In order to assess the effect of release, strontium (Sr) marking method was used to tag chum salmon released in 2016. Sr marking method is that marked and unmarked fish groups were distinguished based on the content of Sr in otolith which was adjusted by changing the Sr concentration of habitat water environment. Sr marking method is widely used in fish enhancement release and achieved many good results (Zhang et al., 2018; Si et al., 2019). Based on the microchemical analysis of otolith samples of chum salmon collectd in Tangwang River by electron probe microanalysis (EPMA). This study intended to reveal whether chum salmon released returned to Tangwang River and it can supply preliminary basic work for the assessment of enhancement release of chum salmon in Tangwang River.
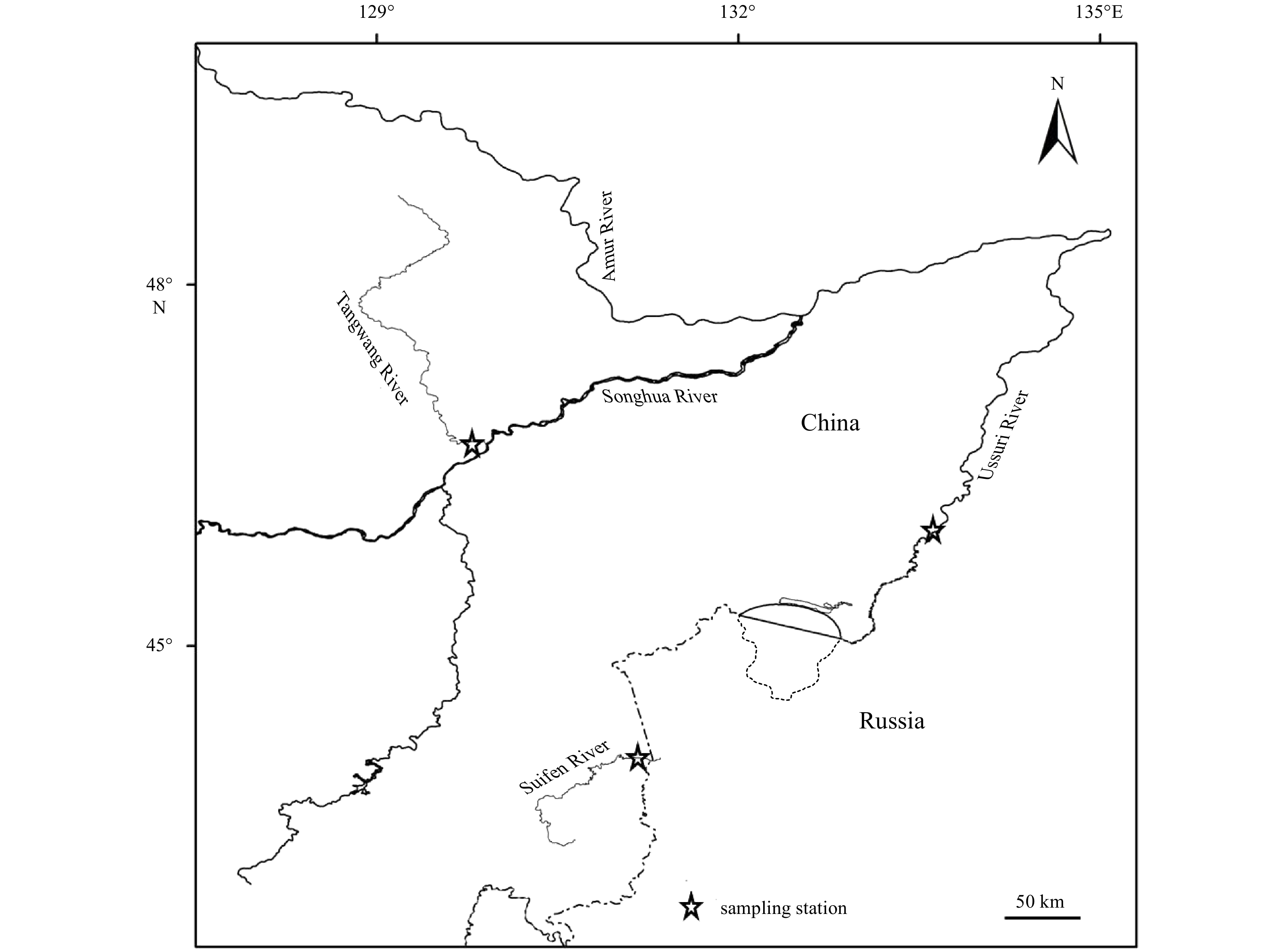
Chum salmon enhancement release in Tangwang River has been conducted since 2012, and the released fishes were tagged by Sr marking method in 2016. The initial concentration of Sr ion in the incubator reached 30 mg/L by adding Sr chloride into hatching water every 12 h for 5 d during the germination of fertilized eggs so as to increase the Sr content of otoliths. The homing chum salmon were detected in Tangwang River, Ussuri River and Suifen River in 2018. A total of 12 specimens (ID: from C1 to C12; fork length ranged from 449 mm to 719 mm; body weight ranged from 1 098 g to 3 902 g) were collected by fishing, of which 4 samples (C1–C4) from Tangyuan section of Tangwang Rvier, other samples were comparative groups made up of 4 samples from Fuyuan section of the Amur River (C5–C8) and 4 samples from Dongning section of Suifen River (C9–C12) respectively (Fig. 1). Chum salmon samples were catched by drift net with the mesh size of 10 cm. Living samples were then sent to the enhancement release station as parent fishes for reproduction at once, after which the otolith samples were collected for further analysis.
The sagittal otoliths of the samples were extracted and cleaned in distilled water, and then dried. Each otolith was embedded in epoxy resin (Epofix, Struers, Ballerup, Denmark), mounted on a glass slide, cut and ground to expose the core on the sagittal plane using a grinding machine equipped with a diamond cup wheel (Discoplan-TS, Struers, Ballerup, Denmark). In order to obtain more exposed surfaces, the sections were further polished with 1 μm diamond paste on an automated polishing wheel (Planopol-35, Struers). Finally, the otoliths were sonicated in Milli-Q water and rinsed with deionized water and dried in an oven at room temperature. For EPMA measurements, all otoliths were fist given a carbon coating with a high vacuum evaporator. The Sr and Ca concentrations along a line drawn on a life-history transect were measured along a line down the longest axis of each otolith from the core to the edge using a wavelength dispersive X-ray electron microprobe (JXA-8900R, JEOL Ltd., Tokyo, Japan). Calcite (CaCO3) and strontianite (SrCO3) were used as standards for the calibration of the Sr and Ca concentrations. The accelerating voltage and beam current were 15 kV and 1.2×10−8 A, respectively. The electron beam was focused on a point 5 μm in diameter, with measurements spaced at 10 μm intervals. X-ray intensity maps of both elements were made of the representative otoliths of the samples using the microprobe in accordance with the results of life-history transects. The beam current was 0.5 μA, counting time was 30 ms, pixel size was 7 μm×7 μm, and the electron beam was focused on a point 5 μm in diameter. The other analytical conditions were consistent with those used for the life history transect analyses (Arai et al., 2003b).
The basic information of chum salmon samples can be seen in Table 1. For the Tangwang Rvier samples, the age of C1 and C4 were 3+ years and they were born in 2015 while C2, C3 were 2+ years and were born in 2016. The age range of other samples is 1+–5+ years. Sr/Ca ratios in otolith transects were high variable among all samples. One dramatic difference between individuals of C2, C3 and other samples was that the maximal Sr/Ca ratio near the otolith core of the former (21.55×1 000 and 20.87×1 000) were dramatically higher than those of the latter (<6×1 000). Except C2 and C3, the Sr/Ca ratio range of the samples is 1.22×1 000–11.55×1 000 which far below the high value area of C2 and C3. So C2 and C3 can be considered as the released population (marked population) based on the result that high value area of otolith stands for the marking area (Fig. 2).
| Samples | Sex | Body fork length/cm | Body weight/g | Age | Sampling river |
| C1 | Female | 65.5 | 2 859.5 | 3+ | Tangwang River |
| C2 | Female | 58.8 | 2 385.2 | 2+ | Tangwang River |
| C3 | Male | 48.9 | 1 019.7 | 2+ | Tangwang River |
| C4 | Female | 58.2 | 1 807.6 | 3+ | Tangwang River |
| C5 | Male | 55.1 | 1 703.7 | 2+ | Ussuri River |
| C6 | Female | 66.2 | 3 013.6 | 4+ | Ussuri River |
| C7 | Female | 69.8 | 3 902.5 | 4+ | Ussuri River |
| C8 | Male | 71.9 | 3 012.3 | 5+ | Ussuri River |
| C9 | Male | 46.3 | 1 205.9 | 1+ | Suifen River |
| C10 | Male | 44.9 | 1 098.2 | 1+ | Suifen River |
| C11 | Male | 49.2 | 1 322.5 | 2+ | Suifen River |
| C12 | Female | 54.9 | 1 761.6 | 2+ | Suifen River |
X-ray intensity maps showed that the Sr varies remarkably among all the samples, although the Ca concentration was similar. For the comparative samples, it showed that the samples have yellowish circular region located at the core. A bluish (low Sr) annular region outside of the core, outer of the bluish region several yellowish and reddish bands can be found alternately (Fig. 3, unmarked sample). However, the reddish annular band which can be considered as the marking ring by Sr was found near the core in C2 and C3 (Fig. 3, marked sample). So it proved that C2 and C3 belonged to the marked released population in Tangwang River in 2016.
The elements in otolith are continuously deposited under the influence of the surrounding water environment, which can preserve the information of the surrounding environment. Therefore, otolith microchemistry is widely used in the study of fish life history (Kotake et al., 2003; Yang et al., 2006; Dou et al. 2012; Beamish et al., 2007). Otolith chemistry can also be used in fish marking to evaluate the effect of enhancement release (Kullmann et al., 2018). Sr element exists widely in nature and the Sr marking method is convenient, safe, and effective (Si et al., 2019; Wang et al., 2015).
Chum salmon belong to typical anadromous migratory species, and they were born and hatched in fresh water until migrating to the sea at the stage of juvenile. Because of the migratory characters, otolith chemistry was widely used in life history study (Arai et al., 2007; Kang et al., 2014; Christian et al., 2013). Because of the difference between the conditions of the fresh water and salt water, the content of Sr and the ratio of Sr/Ca in otolith showed the volatility changes. The values in seawater are generally higher than those in fresh water (Phillis et al., 2011). According to the previous studies, bluish area and the low Sr/Ca ratios region are consistent with a freshwater habitat, while the yellowish, reddish and high Sr/Ca ratios region to the brackish water or seawater in the analysis of EPMA (Arai et al., 2003a, 2003b; Goto and Arai, 2003). Sr in the core of otolith of chum salmon is mainly affected by the yolk sac which was influenced by ambient water during oogenesis (Kang et al., 2014). While Sr is mainly affected by the surrounding freshwater environment from the end of yolk sac absorption to the pre sea stage. So the concentration of Sr in the core region of the otolith of chum salmon is higher than that in its adjacent region. Furthermore physiological factor can also affect the otolith chemistry (Farrell and Campana, 1996). However, the Sr/Ca raito of chum salmon is usualilly smaller than 15×1 000 (1.22×1 000–11.55×1 000 in this study), and in fresh water stage (near the core of otolith) the ratio is much lower than that in salt water (Fig. 3). So the high Sr/Ca ratio of C2 and C3 were caused by Sr marking in the eye stage of fertilized eggs. So it can be proved that C2 and C3 are the tagged individuals released in Tangwang River in 2016. In this study, the marking of chum salmon otolith with the unsteady concentration of strontium ion and initial concentration of Sr ion in incubator is 30 mg/L. The peak value of Sr/Ca ratio is 20×1 000–22×1000 which much lower than the samples tagged by stead concentration (about 77×1 000) (Wang et al., 2015). However, marked and unmarked groups can also been distinguished significantly (Fig. 2).
A total of four chum salmon were collected in the Tangwang River in 2018, of which two of them were born in 2016 and were proved as the released individuals. The other two samples were born in 2015 and it is not known whether they belong to released groups because the chum salmon population released were not marked in 2015. In any case, it has also been shown that the enhancement release of chum salmon in Tangwang River has achieved substantial results, although the number of returned populations is not very large. This confirms the river connectivity in the lower reaches of the Tangwang River and that Tangwang River can be used as a breeding and releasing site of chum salmon to restore its historic salmon habitat and spawning grounds. This study provides a basic data for the population restoration of chum salmon.
We greatly appreciate assistance provided by the fisherman at the three sampling stations in obtaining specimens. We are also grateful to the anonymous reviewers for their valuable suggestions.
| [1] |
Arai T, Goto A, Miyazaki N. 2003a. Use of otolith microchemistry to estimate the migratory history of the threespine stickleback, Gasterosteus aculeatus. Journal of the Marine Biological Association of the United Kingdom, 83(1): 223–230. doi: 10.1017/S0025315403007008h
|
| [2] |
Arai T, Hayano H, Asami H, et al. 2003b. Coexistence of anadromous and lacustrine life histories of the shirauo, Salangichthys microdon. Fisheries Oceanography, 12(2): 134–139. doi: 10.1046/j.1365-2419.2003.00226.x
|
| [3] |
Arai T, Hirata T, Takagi Y. 2007. Application of laser ablation ICPMS to trace the environmental history of chum salmon Oncorhynchus keta. Marine Environmental Research, 63(1): 55–66. doi: 10.1016/j.marenvres.2006.06.003
|
| [4] |
Beamish R J, Neville C M, Sweeting R M, et al. 2007. A proposed life history strategy for the salmon louse, Lepeophtheirus salmonis in the subarctic Pacific. Aquaculture, 264(1–4): 428–440. doi: 10.1016/j.aquaculture.2006.12.039
|
| [5] |
Christian E, Heidi K, Eric C, et al. 2013. Species and life history affect the utility of otolith chemical composition for determining natal stream of origin for pacific salmon. Transactions of the American Fisheries Society, 142(5): 1370–1380. doi: 10.1080/00028487.2013.811102
|
| [6] |
Dou Shuozeng, Yokouchi K, Yu Xin, et al. 2012. The migratory history of anadromous and non-anadromous tapertail anchovy Coilia nasus in the Yangtze River Estuary revealed by the otolith Sr: Ca ratio. Environmental Biology of Fishes, 95(4): 481–490. doi: 10.1007/s10641-012-0042-1
|
| [7] |
Farrell J, Campana S E. 1996. Regulation of calcium and strontium deposition on the otoliths of juvenile tilapia, Oreochromis niloticus. Comparative Biochemistry and Physiology Part A: Physiology, 115(2): 103–109. doi: 10.1016/0300-9629(96)00015-1
|
| [8] |
Goto A, Arai T. 2003. Migratory histories of three types of Cottus pollux (small-egg, middle-egg, and large-egg types) as revealed by otolith microchemistry. Ichthyological Research, 50(1): 67–72. doi: 10.1007/s102280300009
|
| [9] |
Kang S, Kim S, Telmer K, et al. 2014. Stock identification and life history interpretation using trace element signatures in salmon otoliths. Ocean Science Journal, 49(3): 201–210. doi: 10.1007/s12601-014-0020-y
|
| [10] |
Kotake A, Arai T, Ozawa T, et al. 2003. Variation in migratory history of Japanese eels, Anguilla japonica, collected in coastal waters of the Amakusa Islands, Japan, inferred from otolith Sr/Ca ratios. Marine Biology, 142(5): 849–854. doi: 10.1007/s00227-003-1016-9
|
| [11] |
Kullmann B, Hempel M, Thiel R. 2018. Chemical marking of European glass eels Anguilla anguilla with alizarin red S and in combination with strontium: in situ evaluation of short-term salinity effects on survival and efficient mass-marking. Journal of Fish Biology, 92(1): 203–213. doi: 10.1111/jfb.13508
|
| [12] |
Liu Xiaolan. 2011. Effect of the construction of Yichun Hongshan Hydroelectric Power Station on fish resources in Tangwang River. Environmental Science and Management (in Chinese), 36(7): 160–162
|
| [13] |
Phillis C C, Ostrach D J, Ingram B L, et al. 2011. Evaluating otolith Sr/Ca as a tool for reconstructing estuarine habitat use. Canadian Journal of Fisheries and Aquatic Sciences, 68(2): 360–373. doi: 10.1139/F10-152
|
| [14] |
Si Fei, Ren Jiangong, Wang Qinglin, et al. 2019. Strontium marking on otoliths of Paralichthys olivaceus based on immersion experiments. Journal of Fishery Sciences of China (in Chinese), 26(3): 534–545. doi: 10.3724/SP.J.1118.2019.18270
|
| [15] |
Wang Chen, Liu Wei, Zhan Peirong, et al. 2015. Exogenous Sr2+ sedimentation on otolith of chum salmon embryos. Chinese Journal of Applied Ecology (in Chinese), 26(10): 3189–3194
|
| [16] |
Yang Jian, Arai T, Liu Hongbo, et al. 2006. Reconstructing habitat use of Coilia mystus and Coilia ectenes of the Yangtze River estuary, and of Coilia ectenes of Taihu Lake, based on otolith strontium and calcium. Journal of Fish Biology, 69(4): 1120–1135. doi: 10.1111/j.1095-8649.2006.01186.x
|
| [17] |
Yang Fuyi, Yan Baixing, Wang Qiang, et al. 2015. Assessment of fish stocks in the lower reaches of the Songhua River. Wetland Science (in Chinese), 13(1): 87–97
|
| [18] |
Zhang Yi, Jiang Yazhou, Xu Kaida, et al. 2018. Evaluation on effectiveness of marking juvenile Sparus macrocephalus otoliths with strontium. Marine Fisheries (in Chinese), 40(2): 171–178
|
| 1. | Peilun Li, Jiacheng Liu, Wanqiao Lu, et al. Age, growth, reproduction and mortality of Xenocypris argentea (Günther, 1868) in the lower reaches of the Tangwang River, China. PeerJ, 2024, 12: e16673. doi:10.7717/peerj.16673 | |
| 2. | Jilong Wang, Peilun Li, Wei Liu, et al. The Migratory Biology and Feeding Habits of Downstream-Migrating Juvenile Chum Salmon Oncorhynchus keta in the Amur River of Northeast China. Fishes, 2023, 8(9): 458. doi:10.3390/fishes8090458 | |
| 3. | Lingling Gu, Hui Zhang, Guangpeng Feng, et al. Evaluation of the Effectiveness of Marking Juvenile Takifugu obscurus Otoliths with Strontium. Fishes, 2022, 7(6): 371. doi:10.3390/fishes7060371 |

| Samples | Sex | Body fork length/cm | Body weight/g | Age | Sampling river |
| C1 | Female | 65.5 | 2 859.5 | 3+ | Tangwang River |
| C2 | Female | 58.8 | 2 385.2 | 2+ | Tangwang River |
| C3 | Male | 48.9 | 1 019.7 | 2+ | Tangwang River |
| C4 | Female | 58.2 | 1 807.6 | 3+ | Tangwang River |
| C5 | Male | 55.1 | 1 703.7 | 2+ | Ussuri River |
| C6 | Female | 66.2 | 3 013.6 | 4+ | Ussuri River |
| C7 | Female | 69.8 | 3 902.5 | 4+ | Ussuri River |
| C8 | Male | 71.9 | 3 012.3 | 5+ | Ussuri River |
| C9 | Male | 46.3 | 1 205.9 | 1+ | Suifen River |
| C10 | Male | 44.9 | 1 098.2 | 1+ | Suifen River |
| C11 | Male | 49.2 | 1 322.5 | 2+ | Suifen River |
| C12 | Female | 54.9 | 1 761.6 | 2+ | Suifen River |