| Citation: | Yanbin Tang, Qiang Liu, Yibo Liao, Konglin Zhou, Lu Shou. Responses of macrobenthic communities to patchy distributions of heavy metals and petroleum hydrocarbons in sediments: A study in China’s Zhoushan Archipelago[J]. Acta Oceanologica Sinica, 2021, 40(9): 117-125. doi: 10.1007/s13131-021-1892-1 |
The family Nassariidae is a relatively diverse group of buccinoid gastropods occurring worldwide, with a wide habitat range encompassing temperate, subtropical and tropical waters, from the intertidal zone to deeper waters (0–1000 m depth, mostly 0–300 m) (Cernohorsky, 1972, 1984). Some species have even invaded freshwater (Strong et al., 2017 and references therein). In recent years, intensive efforts to understand the evolutionary relationships of the Buccinoidea have resulted in significant advances towards a stable classification of Nassariidae (Galindo et al., 2016); as a part of these efforts, some genera previously included in Buccinidae were transferred to the Nassariidae. Currently, the family Nassariidae includes more than 600 species in at least 23 genera (MolluscaBase, 2020).
In China, species and fauna of Nassariidae have been investigated by various authors (e.g., Zhang, 2009, 2010; Yang, 2010; Zhang and Yang, 2010), with more than 70 species have been recorded. However, the biodiversity of this group is still underestimated. Increased taxonomic efforts over recent years have revealed several undescribed or unrecorded nassariid species in China (Zhang, 2013; Zhang and Zhang, 2014; Zhang and Zhang, 2018; Zhang et al., 2019).
Recently, examination of nassariid specimens preserved at the Marine Biological Museum of Chinese Academy of Sciences (MBMCAS), Qingdao revealed two new species belonging to the genus Nassarius. In the present paper, we formally describe and illustrate these two species to increase the taxonomic knowledge of Nassariidae from Chinese waters.
Specimens of Nassarius nanshaensis sp. nov. were collected by Agassiz trawl from shallow water (56–147 m) off the Nansha Islands; and the specimens of Nassarius concavus sp. nov. were sampled from the South China Sea at a depth of 180 m. Two specimens of Nassarius nanshaensis sp. nov., including the holotype, were dissected for examination of radulae. Shells and operculum were observed by a light microscope and radulae by scanning electron microscope (SEM). For SEM, radulae were placed in 10% NaOH for 2 h, washed in distilled water, then laid on a cover slip to air-dry. Finally, the samples were coated with gold and examined under SEM.
The following abbreviations are used in the text: CN, collection number; MBMCAS, Marine Biological Museum of Chinese Academy of Sciences, Qingdao; RN, registration number; St, station; spm(s), specimen(s); coll(s)., collector(s).
Class Gastropoda Cuvier, 1795
Superfamily Buccinoidea Rafinesque, 1815
Family Nassariidae Iredale, 1916 (1835)
Genus Nassarius Duméril, 1805
Type species: Buccinum arcularia Linnaeus, 1758, by subsequent designation. Recent, Indo-Pacific Ocean.
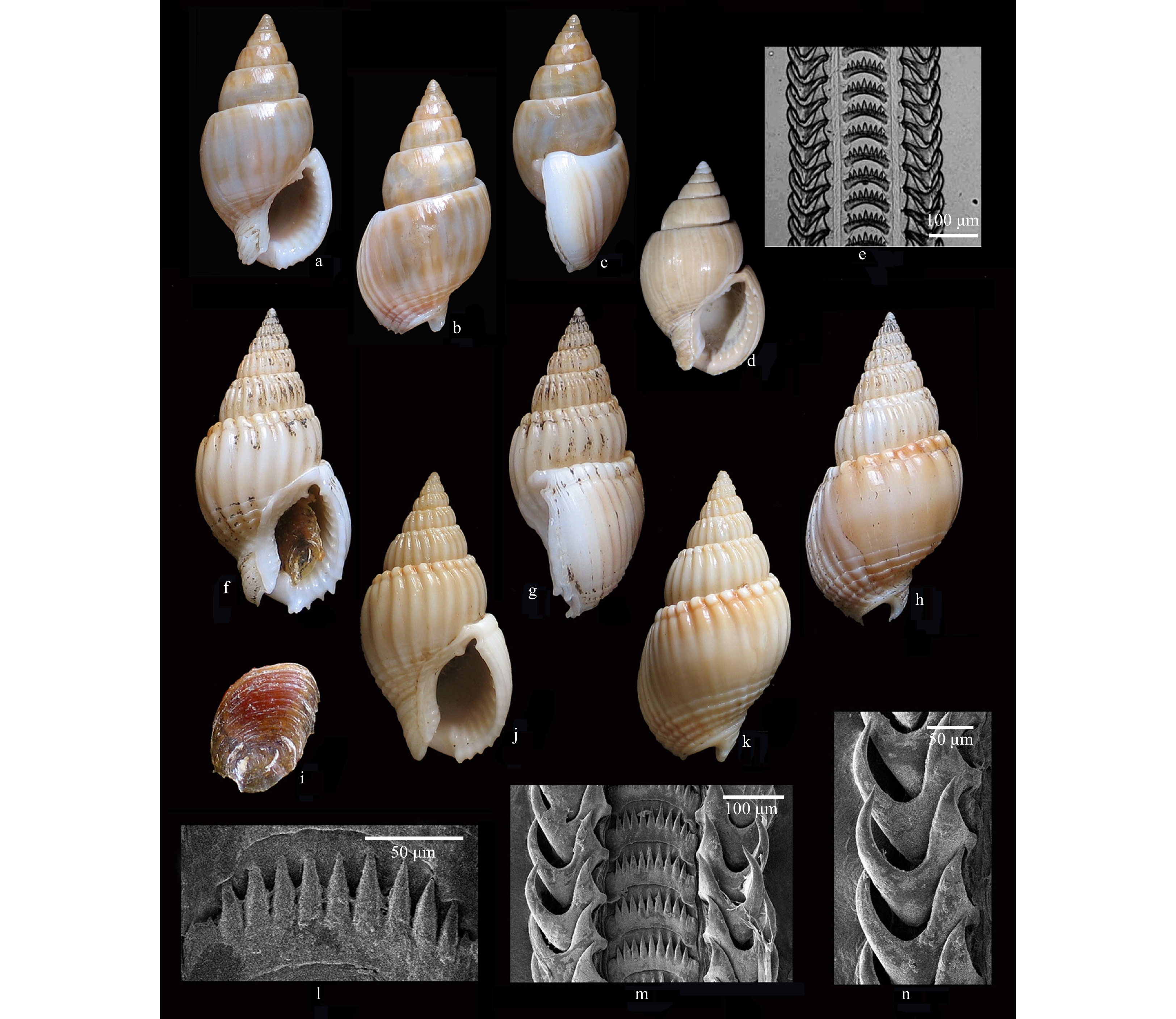
Nassarius concavus sp. nov. (Figs 1a–e)

Nassarius (Zeuxis) sp. Li et al., 2010 (Figs 1f, h)
Material examined: Holotype, RN: MBM286692 (15.6 mm in length, 7.8 mm in width), South China Sea, off Guangdong Province (20°52′N, 114°53′E), sandy bottom, 180 m, in MBMCAS, Qingdao.
Paratype: 1 spm, RN: MBM286693 (12.0 mm in length, 6.8 mm in width), collected together with the holotype.
Description of the holotype: Shell (Figs 1a–d) thin, semitransparent, of medium size for the genus; last whorl large, 2/3 of the shell length. Protoconch of 3.25 glassy whorls; last 1.25 whorls angulated with a distinct peripheral keel. Transition to teleoconch distinct. Teleoconch of 6 evenly convex whorls. Suture deeply channeled. First teleoconch whorl with weak axial waves, probably due to uneven thickness of material in underlying shell layers or irregular erosion. Subsutural groove on first two teleoconch whorls. Subsequent whorls smooth except for microscopic axial striae. Aperture slightly higher than the spire, outer lip variced, interior with 11–12 moderately long lirae, outer lip anteriorly with 4 indistinct, pointed denticles. Columella arched, upper region with a prominent parietal tooth. Columellar callus thin and narrowly margined. Siphonal canal short with a prominent, U-shaped notch. Anal canal narrow. Shell color whitish to light yellow, with prominent brownish longitudinal streaks.
Radula: Central tooth evenly arched, posterior margin with 9 long, sharp-pointed denticles. Lateral teeth with 2 large, sharp-pointed cusps, outer one longer and narrower than the inner one (Fig. 1e).
Etymology: The species is named concavus in reference to the concave shape of the suture.
Type locality: South China Sea, off Guangdong Province, China.
Distribution and habitat: Only known from the type locality, South China Sea, off Guangdong Province, where they live on sandy bottom in 180 m depth.
Remarks: Nassarius concavus sp. nov. was illustrated as Nassarius (Zeuxis) sp. by Li et al. (2010) (Figs 1f, h). In that publication, they also mentioned another species, Nassarius (Zeuxis) algidus (Reeve, 1853) (Figs 1g, i), which was subsequently described as Nassarius glabrus Zhang and Zhang, 2014. The two species are very similar in general shell morphology; however, Nassarius glabrus differs from Nassarius concavus sp. nov. in having a large, broader shell with well-developed axial ribs on the first 2 to 3 teleoconch whorls. In addition, the molecular analysis based on the sequences of the mtCOI gene confirmed that they represent two different species (Li et al., 2010).
Nassarius excellens (Kuroda and Habe, 1961) (Habe, 1961) and Nassarius tangaroai Kool, 2006 resemble Nassarius concavus sp. nov. in shell shape and color pattern, but they can be distinguished from the new species in having axial ribs on earlier teleoconch whorls, a subsutural groove and a non-channeled suture.
Nassarius concavus sp. nov. may be confused with Nassarius kooli Dekker and Dekkers, 2009 because of the similarity in shell shape and in possession of a deeply channeled suture. Nassarius kooli differs from the new species in having prominent axial ribs on earlier teleoconch whorls, a subsutural groove, numerous microscopic spirals and a different color pattern (Dekker and Dekkers, 2009; Zhang and Zhang, 2014).
Nassarius nanshaensis sp. nov. (Figs 1f–n)
Material examined: Holotype, RN: MBM286694, St: 64 (4°00′N, 112°06′E), CN: SSBV27–15, Nansha Islands, muddy sand, 56 m, 1 August 1988, coll. Ruiqiu Chen, in MBMCAS.
Paratypes: 1 spm, RN: MBM286695, CN: SSBV26-33, St: 60 (5°20′N, 112°06′E), Nansha Islands, muddy sand, 127 m, 31 July 1988, coll. Ruiqiu Chen; 5 spms, RN: MBM286696, CN: SSIVB50-11, St: 50 (5°38′N, 109°05′E), Nansha Islands, 147 m, 16 May 1987, coll. Ruiqiu Chen; 2 spms, RN: MBM286697, CN: SSBIV-4, St: 29 (6°00′N, 112°15′E), Nansha Islands, soft mud, 105 m, 11 May 1987, coll. Ruiqiu Chen; 2 spms, RN: MBM286698, CN: SSIVB40-11, St: 49 (5°15′N, 109°45′E), muddy sand, 111 m, 16 May 1987, coll. Ruiqiu Chen; 6 spms, RN: MBM286699, CN: SSB10-6, St: 64 (4°30′N, 110°30′E), Nansha Islands, sand and mud, 100 m, 23 September 1994, colls. Zhican Tang, Xianqiu Ren; 5 spms, RN: MBM286700, CN: SSB12-7, St: 68 (5°00′ N, 112°00′ E), Nansha Islands, 102 m, 23 September 1994; colls. Zhican Tang, Xianqiu Ren.
Description of the holotype: Shell (Figs 1f–h, j and k) thick, solid, of medium size for the genus; spire acute; last whorl large, 2/3 of shell length. Protoconch of 2.5 glassy, slightly angulated whorls with a distinct keel on the middle part, transition to teleoconch distinct. Teleoconch of 6 evenly convex whorls. Suture ledged, slightly channeled. First 4 teleoconch whorls sculptured with weak, wide, flattened spiral cords, numbering 6–7 on the antepenultimate whorl. Each teleoconch whorl with a distinct subsutural spiral groove. Spiral sculpture gradually becoming reduced and absent on penultimate whorl and upper part of the last whorl; shell base with 4–5 deep grooves. Axial ribs thin and narrowly spaced on upper whorls (24–25 on penultimate whorl), becoming broader on ventral surface of last whorl, reducing on the dorsal surface of last whorl and finally recurring towards the aperture. Aperture as long as spire, outer lip slightly variced, edge sharp, interior with 14 moderately long lirae, outer lip anteriorly with 5–6 small, pointed denticles. Columella arched, upper region with a prominent parietal tooth. Columellar callus well developed and margined. Siphonal canal narrow with a prominent, U-shaped notch. Anal canal narrow. Shell color whitish to yellowish brown, outer lip and columella white, aperture orange-yellowish inside, brownish spiral band vague on spire, visible on dorsal surface of the body whorl.
Operculum (Fig. 1i) ovate, brownish, inner margin smooth, outer margin with 4–5 prominent serrations.
Radula (Figs 1l–n): Central tooth evenly arched, posterior margin with 9–11 long, sharp-pointed denticles. Lateral teeth with 2 large, sharp-pointed cusps, outer one longer and narrower than the inner one.
Variability: The adult size of the shells varies from about 16.5 mm to 25.2 mm, most 21.0–23.0 mm. The colour varies from creamy white to light browish, with vague brownish bands. The axial ribs on dorsal surface of the last whorl are weak or absent.
Etymology: The new species is named after its type locality, Nansha Islands.
Type locality: Nansha Islands, South China Sea, China.
Distribution and habitat: To date only known from Nansha Islands, where they live on muddy or sandy bottom at depth range of 56–147 m.
Measurements: See Table 1.
| Holotype | Paratype 1 | Paratype 2 | Paratype 3 | Paratype 4 | |
| Shell length | 25.2 | 24.2 | 22.3 | 21.5 | 18.5 |
| Shell width | 12.3 | 12.0 | 11.5 | 10.8 | 10.4 |
Remarks: To date, more than 70 species of the genus Nassarius have been recorded from Chinese waters. Of these, only two species, Nassarius multivocus Kool, 2008 and Nassarius maxiutongi Zhang, Zhang and Li, 2019, could be confused with Nassarius nanshaensis sp. nov. in terms of the shell size and general shape. However, Nassarius multivocus can be separated from the new species on the basis of the presence of sculpture over the whole shell surface (Kool, 2008; Zhang et al., 2019). Nassarius maxiutongi has a similar sculpture to Nassarius nanshaensis sp. nov., but differs in having a broader shell, thicker axial ribs, and a lower number of cusps on the rachidian tooth (9–11 vs. 13–17).
In China, the family Nassariidae was systematically studied by Yang (2010) based on the shell and radula. As a result, a total of 46 species in two genera were recorded. Since that time, extensive taxonomic efforts have revealed many new species and new records from Chinese waters (Zhang, 2009, 2010, 2013; Zhang and Zhang, 2014; Zhang and Zhang, 2018; Zhang et al., 2019). There are at least 100 nassariid species in Chinese waters (authors’ unpublished data), a much higher number than previously thought. These findings strongly suggest that the biodiversity of Nassariidae in Chinese waters is still underestimated, and there are more species waiting to be discovered.
Accurate identification and delimitation of species are essential for assessing biodiversity; however, previous descriptions of nassariid species were mainly based on their shells, which sometimes do not provide sufficient morphological information, resulting in taxonomic confusion and, in turn, in an inaccurate estimate of biodiversity. The morphology of the radula is typically considered to be an important feature for gastropod taxonomy. Some authors have emphasized that the radular morphology of Nassariidae can be used as a basis for both genus and species distinctions (Habe, 1964; Adams and Knudsen, 1984), but others noted that radulae seem not to be practical for taxonomy, especially for species delimitation (Troschel, 1867; Cernohorsky, 1984). Yang and Zhang (2011) examined the radulae of 22 nassariid species from China in detail and concluded that radula morphology could be used to distinguish species. In recent years, some molecular studies of Nassariidae have been conducted in China based on mitochondrial and/or nuclear genes (Li et al., 2010; Chen and Zhang, 2012; Zou et al., 2012). Although those molecular studies determined some systematic relationships among species and revealed several cryptic species, many species names used were misidentifications that resulted in inconsistent conclusions. In the future, traditional shell-based identification should, where possible, be combined with radular and molecular features to ensure accurate taxonomy and classification of Nassariidae.
We are grateful to all the collectors for their great efforts in sample collection.
| [1] |
Adeleye A O, Jin Haiyan, Di Ya’nan, et al. 2016. Distribution and ecological risk of organic pollutants in the sediments and seafood of Yangtze Estuary and Hangzhou Bay, East China Sea. Science of the Total Environment, 541: 1540–1548. doi: 10.1016/j.scitotenv.2015.09.124
|
| [2] |
Anger K. 2003. Salinity as a key parameter in the larval biology of decapod crustaceans. Invertebrate Reproduction & Development, 43(1): 29–45
|
| [3] |
Barrio Froján C R S, Boyd S E, Cooper K M, et al. 2008. Long-term benthic responses to sustained disturbance by aggregate extraction in an area off the east coast of the United Kingdom. Estuarine, 79(2): 204–212
|
| [4] |
Bastami K D, Bagheri H, Kheirabadi V, et al. 2014. Distribution and ecological risk assessment of heavy metals in surface sediments along southeast coast of the Caspian Sea. Marine Pollution Bulletin, 81(1): 262–267. doi: 10.1016/j.marpolbul.2014.01.029
|
| [5] |
Braeckman U, Foshtomi M Y, Van Gansbeke D, et al. 2014. Variable importance of macrofaunal functional biodiversity for biogeochemical cycling in temperate coastal sediments. Ecosystems, 17(4): 720–737
|
| [6] |
Bryan G W, Langston W J. 1992. Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environmental Pollution, 76(2): 89–131. doi: 10.1016/0269-7491(92)90099-V
|
| [7] |
Carvalho R, Wei C L, Rowe G, et al. 2013. Complex depth-related patterns in taxonomic and functional diversity of polychaetes in the Gulf of Mexico. Deep Sea Research Part I: Oceanographic Research Papers, 80: 66–77. doi: 10.1016/j.dsr.2013.07.002
|
| [8] |
Chai Xiaoping, Hu Baolan, Wei Na, et al. 2015. Distribution, sources and assessment of heavy metals in surface sediments of the Hangzhou Bay and its adjacent areas. Acta Scientiae Circumstantiae (in Chinese), 35(12): 3906–3916
|
| [9] |
Che Yue, He Qing, Lin Weiqing. 2003. The distributions of particulate heavy metals and its indication to the transfer of sediments in the Changjiang Estuary and Hangzhou Bay, China. Marine Pollution Bulletin, 46(1): 123–131. doi: 10.1016/S0025-326X(02)00355-7
|
| [10] |
Cibic T, Franzo A, Celussi M, et al. 2012. Benthic ecosystem functioning in hydrocarbon and heavy-metal contaminated sediments of an Adriatic lagoon. Marine Ecology Progress Series, 458: 69–87. doi: 10.3354/meps09741
|
| [11] |
Dauer D M, Ranasinghe J A, Weisberg S B. 2000. Relationships between benthic community condition, water quality, sediment quality, nutrient loads, and land use patterns in Chesapeake Bay. Estuaries, 23(1): 80–96. doi: 10.2307/1353227
|
| [12] |
Dauvin J C. 2007. Paradox of estuarine quality: Benthic indicators and indices, consensus or debate for the future. Marine Pollution Bulletin, 55(1–6): 271–281
|
| [13] |
Delta M. 1990. Marine transport and deposition. Developments in Sedimentology, 44(100): 279–343
|
| [14] |
Dong Aiguo, Zhai Shikui, Zabel M, et al. 2012. Heavy metals in Changjiang estuarine and offshore sediments: responding to human activities. Acta Oceanologica Sinica, 31(2): 88–101. doi: 10.1007/s13131-012-0195-y
|
| [15] |
Eleftheriou A, McIntyre A D. 2005. Methods for the Study of Marine Benthos. 3rd ed. Oxford: Blackwell Science, 10–11
|
| [16] |
Fang Hongwei, Huang Lei, Wang Jingyu, et al. 2016. Environmental assessment of heavy metal transport and transformation in the Hangzhou Bay, China. Journal of Hazardous Materials, 302: 447–457. doi: 10.1016/j.jhazmat.2015.09.060
|
| [17] |
Fang Ming, Wu Youjun, Liu Hong, et al. 2013. Distribution, sources and ecological risk assessment of heavy metals in sediments of the Yangtze River Estuary. Acta Scientiae Circumstantiae (in Chinese), 33(2): 563–569
|
| [18] |
Fernandes M B, Sicre M A, Boireau A, et al. 1997. Polyaromatic hydrocarbon (PAH) distributions in the Seine River and its estuary. Marine Pollution Bulletin, 34(11): 857–867. doi: 10.1016/S0025-326X(97)00063-5
|
| [19] |
Frid C L J, Caswell B A. 2015. Is long-term ecological functioning stable: the case of the marine benthos?. Journal of Sea Research, 98: 15–23. doi: 10.1016/j.seares.2014.08.003
|
| [20] |
Gao Xuelu, Chen Shaoyong. 2008. Petroleum pollution in surface sediments of Daya Bay, South China, revealed by chemical fingerprinting of aliphatic and alicyclic hydrocarbons. Estuarine, 80(1): 95–102
|
| [21] |
Ghribi R, Correia A T, Elleuch B, et al. 2019. Toxicity assessment of impacted sediments from southeast coast of Tunisia using a biomarker approach with the polychaete Hediste diversicolor. Archives of Environmental Contamination and Toxicology, 76(4): 678–691. doi: 10.1007/s00244-019-00611-2
|
| [22] |
Giangrande A, Licciano M, Musco L. 2005. Polychaetes as environmental indicators revisited. Marine Pollution Bulletin, 50(11): 1153–1162. doi: 10.1016/j.marpolbul.2005.08.003
|
| [23] |
Goh C P, Lim P E. 2008. Potassium permanganate as oxidant in the cod test for saline water samples. ASEAN Journal on Science and Technology for Development, 25(2): 383–393
|
| [24] |
Gopalakrishnan S, Thilagam H, Raja P V. 2008. Comparison of heavy metal toxicity in life stages (spermiotoxicity, egg toxicity, embryotoxicity and larval toxicity) of Hydroides elegans. Chemosphere, 71(3): 515–528. doi: 10.1016/j.chemosphere.2007.09.062
|
| [25] |
Hakanson L. 1980. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14(8): 975–1001. doi: 10.1016/0043-1354(80)90143-8
|
| [26] |
Hu Rijun, Wu Jianzheng, Li Guangxue, et al. 2009. Characteristics of sediment transport in the Zhoushan Archipelago sea area. Acta Oceanologica Sinica, 28(5): 116–127
|
| [27] |
Hunter W R, Levin L A, Kitazato H, et al. 2012. Macrobenthic assemblage structure and organismal stoichiometry control faunal processing of particulate organic carbon and nitrogen in oxygen minimum zone sediments. Biogeosciences, 9(3): 993–1006. doi: 10.5194/bg-9-993-2012
|
| [28] |
Ji Weidong. 2011. Research on the Current Status and Background Value of China’s Offshore Marine Environmental Quality (in Chinese). Beijing: China Ocean Press, 161–164
|
| [29] |
Jiang H, Hu Y, Xu L, et al. 2011. Evaluation on pollution and potential ecological risk of 5 heavy metals in the surface sediments of Zhoushan coastal area. Journal of Marine Sciences (in Chinese), 29(1): 1116–1118
|
| [30] |
Johnston E L, Roberts D A. 2009. Contaminants reduce the richness and evenness of marine communities: a review and meta-analysis. Environmental Pollution, 157(6): 1745–1752. doi: 10.1016/j.envpol.2009.02.017
|
| [31] |
Jović M, Stanković S. 2014. Human exposure to trace metals and possible public health risks via consumption of mussels Mytilus galloprovincialis from the Adriatic coastal area. Food and Chemical Toxicology, 70: 241–251. doi: 10.1016/j.fct.2014.05.012
|
| [32] |
Li Haiming, Zheng Xilai, Liu Xianbing. 2005. Regulation of movement and transformation of oil in sediment on tidal flat of Bohai Bay. Marine Environmental Science (in Chinese), 24(3): 9–12
|
| [33] |
Liu Peizhe, Yu Yongquan, Liu Chunyu. 1991. Studies on the situation of pollution and countermeasures of control of the oceanic environment in Zhoushan fishing ground-the largest fishing ground in China. Marine Pollution Bulletin, 23: 281–288. doi: 10.1016/0025-326X(91)90688-O
|
| [34] |
Loder A L, Mallory M L, Spooner I, et al. 2016. Bioaccumulation of lead and arsenic in gastropods inhabiting salt marsh ponds in coastal bay of Fundy, Canada. Water, Air, & Soil Pollution, 227(3): 75
|
| [35] |
Long W C, Brylawski B J, Seitz R D. 2008. Behavioral effects of low dissolved oxygen on the bivalve Macoma balthica. Journal of Experimental Marine Biology and Ecology, 359(1): 34–39. doi: 10.1016/j.jembe.2008.02.013
|
| [36] |
Ma Jiayi. 2012. Research of risk assessment on oil spill accidents by ship. Journal of Zhejiang Ocean University: Natural Science (in Chinese), 31(2): 182–187
|
| [37] |
Monte L. 2002. A methodology for modelling the contamination of moving organisms in water bodies with spatial and time dependent pollution levels. Ecological Modelling, 158(1–2): 21–33
|
| [38] |
Mucha A P, Vasconcelos M T S D, Bordalo A A. 2003. Macrobenthic community in the Douro estuary: Relations with trace metals and natural sediment characteristics. Environmental Pollution, 121(2): 169–180. doi: 10.1016/S0269-7491(02)00229-4
|
| [39] |
Obolewski K, Glińska-Lewczuk K, Szymańska M, et al. 2018. Patterns of salinity regime in coastal lakes based on structure of benthic invertebrates. PLoS One, 13(11): e0207825. doi: 10.1371/journal.pone.0207825
|
| [40] |
Parmar T K, Rawtani D, Agrawal Y K. 2016. Bioindicators: the natural indicator of environmental pollution. Frontiers in Life Science, 9(2): 110–118. doi: 10.1080/21553769.2016.1162753
|
| [41] |
Pearson T H, Rosenberg R. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology: An Annual Review, 16: 229–311
|
| [42] |
Pechenik J A, Marsden I D, Pechenik O. 2003. Effects of temperature, salinity, and air exposure on development of the estuarine pulmonate gastropod Amphibola crenata. Journal of Experimental Marine Biology and Ecology, 292(2): 159–176. doi: 10.1016/S0022-0981(03)00159-X
|
| [43] |
Rouse G W, Pleijel F. 2001. Polychaetes. Oxford: Oxford University Press
|
| [44] |
Rowlatt S M, Lovell D R. 1994. Lead, zinc and chromium in sediments around England and Wales. Marine Pollution Bulletin, 28(5): 324–329. doi: 10.1016/0025-326X(94)90159-7
|
| [45] |
Rumisha C, Elskens M, Leermakers M, et al. 2012. Trace metal pollution and its influence on the community structure of soft bottom molluscs in intertidal areas of the Dar es Salaam coast, Tanzania. Marine Pollution Bulletin, 64(3): 521–531. doi: 10.1016/j.marpolbul.2011.12.025
|
| [46] |
Ryu J, Khim J S, Kang S G, et al. 2011. The impact of heavy metal pollution gradients in sediments on benthic macrofauna at population and community levels. Environmental Pollution, 159(10): 2622–2629. doi: 10.1016/j.envpol.2011.05.034
|
| [47] |
Shannon C E, Weaver W. 1964. The mathematical theory of communication. Urbana: The University of Illinois Press, 117
|
| [48] |
Sharma V K, Sohn M. 2009. Aquatic arsenic: toxicity, speciation, transformations, and remediation. Environment International, 35(4): 743–759. doi: 10.1016/j.envint.2009.01.005
|
| [49] |
Shou Lu, Huang Yijun, Zeng Jiangning, et al. 2009. Seasonal changes of macrobenthos distribution and diversity in Zhoushan sea area. Aquatic Ecosystem Health & Management, 12(1): 110–115
|
| [50] |
Thompson B, Lowe S. 2004. Assessment of macrobenthos response to sediment contamination in the San Francisco Estuary, California, USA. Environmental Toxicology and Chemistry, 23(9): 2178–2187. doi: 10.1897/03-233
|
| [51] |
Tong Yifan, Li Jingyi, Cheng Qianhui, et al. 2019. Enhanced removal of sediment-associated total petroleum hydrocarbons under bioturbation by polychaete Perinereis aibuhitensis. Journal of Environmental Science and Health, Part A, 54(5): 391–397. doi: 10.1080/10934529.2018.1558894
|
| [52] |
UNEP. 1992. Determination of petroleum hydrocarbons in sediments. In: Reference Methods for Marine Pollution Studies No. 20. United Nations: UNEP, 28–30.
|
| [53] |
Van Hoey G, Borja A, Birchenough S, et al. 2010. The use of benthic indicators in Europe: from the water framework directive to the marine strategy framework directive. Marine Pollution Bulletin, 60(12): 2187–2196. doi: 10.1016/j.marpolbul.2010.09.015
|
| [54] |
van Oevelen D, Soetaert K, Middelburg J J, et al. 2006. Carbon flows through a benthic food web: Integrating biomass, isotope and tracer data. Journal of Marine Research, 64(3): 453–482. doi: 10.1357/002224006778189581
|
| [55] |
Varjani S J. 2017. Microbial degradation of petroleum hydrocarbons. Bioresource Technology, 223: 277–286. doi: 10.1016/j.biortech.2016.10.037
|
| [56] |
Wang Jieyu, Yu Xinwei, Fang Li. 2014. Organochlorine pesticide content and distribution in coastal seafoods in Zhoushan, Zhejiang Province. Marine Pollution Bulletin, 80(1–2): 288–292
|
| [57] |
Wang Xiaoyan, Xu Huanzhi, Zhou Yongdong, et al. 2015. Distribution and source apportionment of polycyclic aromatic hydrocarbons in surface sediments from Zhoushan Archipelago and Xiangshan Harbor, East China Sea. Marine Pollution Bulletin, 101(2): 895–902. doi: 10.1016/j.marpolbul.2015.10.073
|
| [58] |
Warwick R M, Clarke K R. 1984. Species size distributions in marine benthic communities. Oecologia, 61(1): 32–41. doi: 10.1007/BF00379085
|
| [59] |
Weston D P. 1990. Quantitative examination of macrobenthic community changes along an organic enrichment gradient. Marine Ecology Progress Series, 61: 233–244. doi: 10.3354/meps061233
|
| [60] |
Xu Fanglu, Ji Zhongqiang, Wang Kui, et al. 2016. The distribution of sedimentary organic matter and implication of its transfer from Changjiang Estuary to Hangzhou Bay, China. Open Journal of Marine Science, 6(1): 103–114. doi: 10.4236/ojms.2016.61010
|
| [61] |
Xu Na’na, Qiu Ying, Yao Yanming, et al. 2015. Ecological environment changes in Zhoushan coastal waters from 2002 to 2011. Journal of Anhui Agricultural Sciences (in Chinese), 43(3): 292–296
|
| [62] |
Xue Jianliang, Yu Yang, Bai Yu, et al. 2015. Marine oil-degrading microorganisms and biodegradation process of petroleum hydrocarbon in marine environments: a review. Current Microbiology, 71(2): 220–228. doi: 10.1007/s00284-015-0825-7
|
| [63] |
Yılmaz A B, Yanar A, Alkan E N. 2017. Review of heavy metal accumulation on aquatic environment in Northern East Mediterrenean Sea part I: some essential metals. Reviews on Environmental Health, 32(1–2): 119–163
|
| [64] |
Yokoyama H. 1988. Effects of temperature on the feeding activity and growth rate of the spionid polychaete Paraprionospio sp. (form A). Journal of Experimental Marine Biology and Ecology, 123(1): 41–60. doi: 10.1016/0022-0981(88)90108-6
|
| [65] |
Zhang Weiyan, Jin Haiyan, Yao Xuying, et al. 2015. Grain size composition and transport of sedimentary organic carbon in the Changjiang River (Yangtze River) Estuary and Hangzhou Bay and their adjacent waters. Acta Oceanologica Sinica, 34(10): 46–56. doi: 10.1007/s13131-015-0711-y
|
| [66] |
Zhang Le, Zhu Baikang. 2013. The current situation of oil spill processing and recycling technical analysis in Zhoushan Harbor. Journal of Zhejiang Ocean University: Natural Science (in Chinese), 32(1): 81–85
|
| [67] |
Zhuo Lifei, Li Zimeng, Jin Yanjian. 2019. A study of particle size and heavy metals in surface sediments of Zhoushan region. Marine Environmental Science (in Chinese), 38(1): 52–59
|
| 1. | Suping Zhang, Shuqian Zhang. Descriptions of two species of Nassarius (Gastropoda: Nassariidae) from the South China Sea. Journal of Oceanology and Limnology, 2023. doi:10.1007/s00343-022-1451-4 |

| Holotype | Paratype 1 | Paratype 2 | Paratype 3 | Paratype 4 | |
| Shell length | 25.2 | 24.2 | 22.3 | 21.5 | 18.5 |
| Shell width | 12.3 | 12.0 | 11.5 | 10.8 | 10.4 |