The decomposition rate of the organic carbon content of suspended particulate matter in the tropical seagrass meadows
-
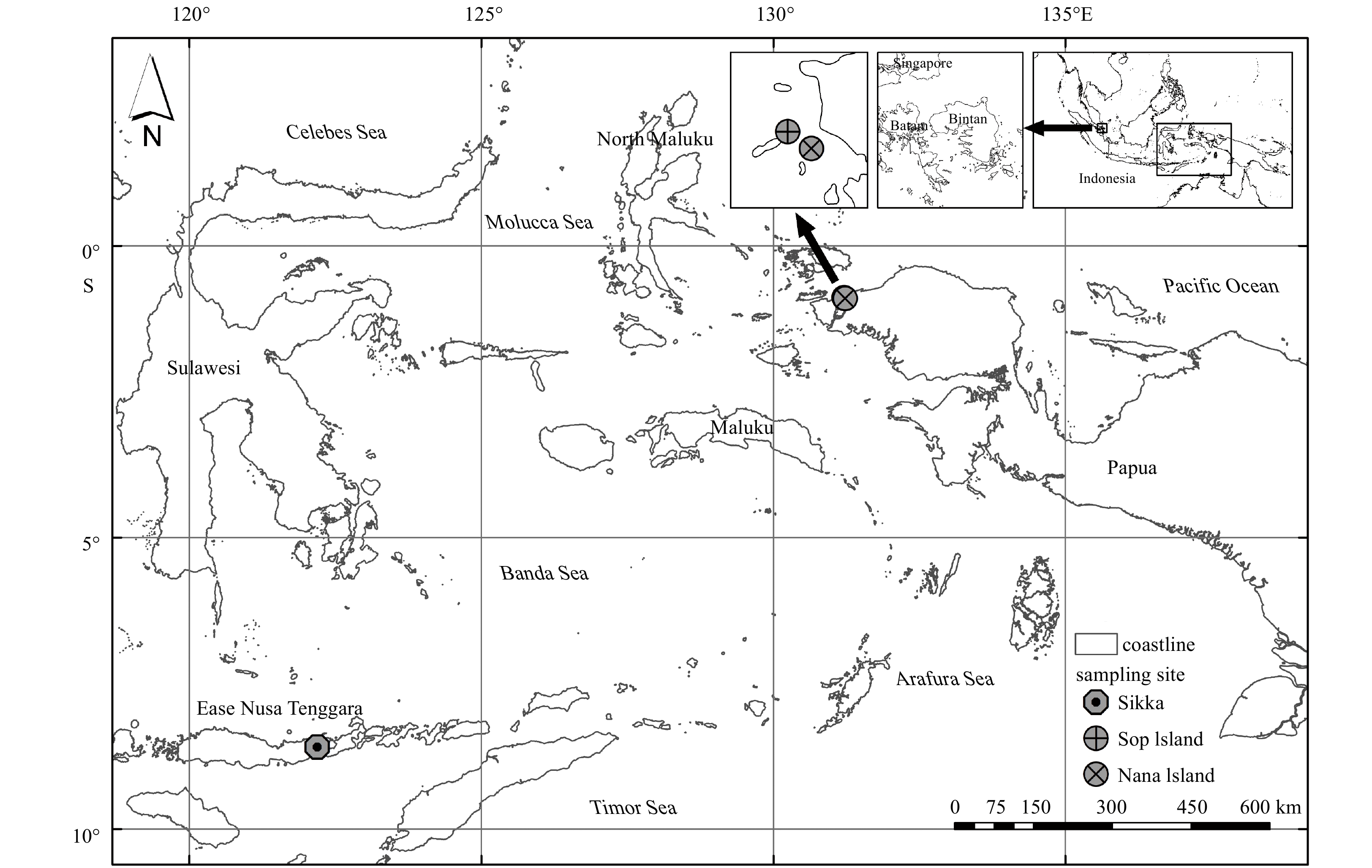
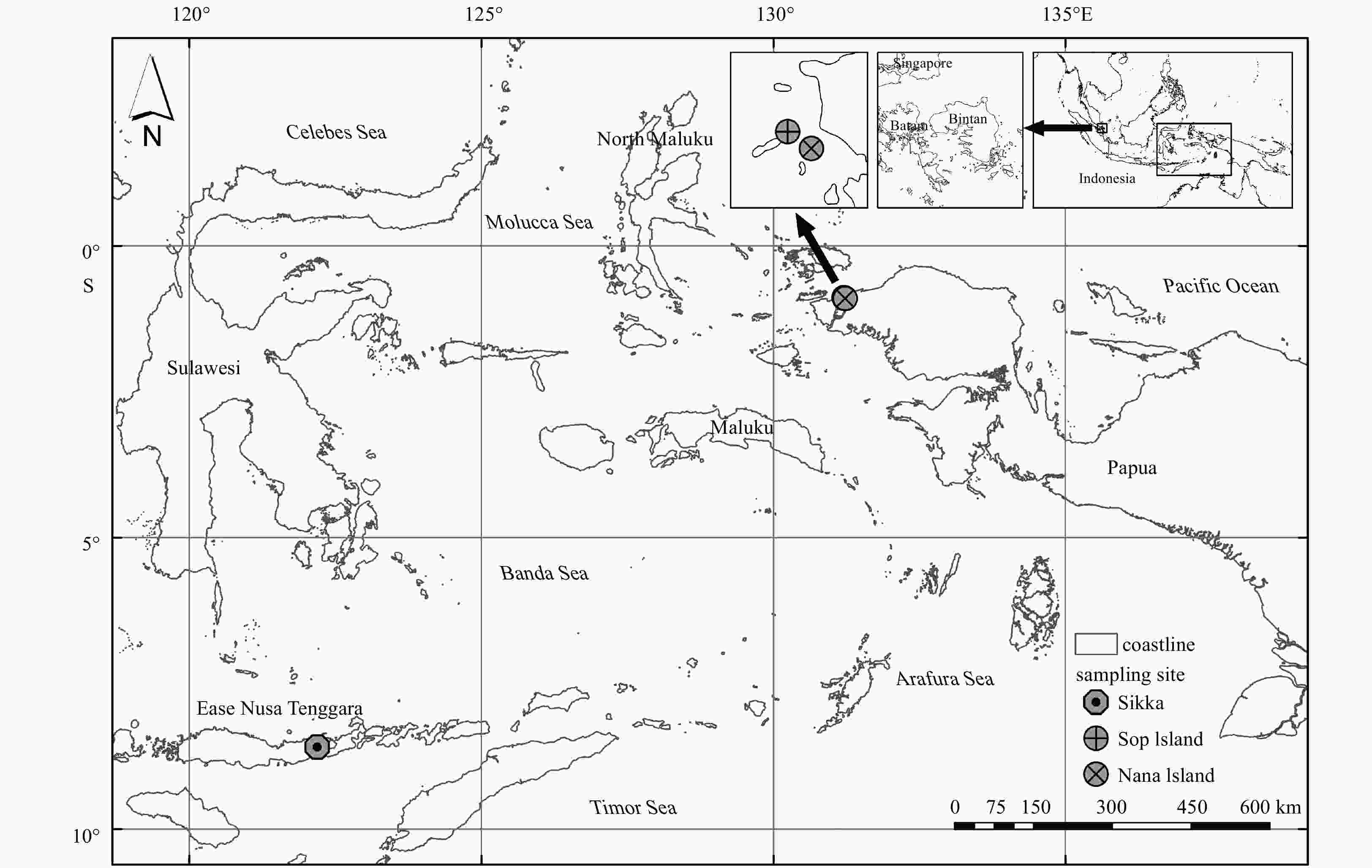
Abstract: In terms of downward transport, suspended particulate matter (SPM) from marine or terrigenous sources is an essential contributor to the carbon cycle. Within mesoscale environments such as seagrass ecosystems, SPM flux is an essential part of the total carbon budget that is transported within the ecosystem. By assessing the total SPM transport from water column to sediment, potential carbon burial can be estimated. However, SPM may decompose or reforming aggregate during transport, so estimating the vertical flux without knowing the decomposition rate will lead to over- or underestimation of the total carbon budget. Here this paper presents the potential decomposition rate of the SPM in seagrass ecosystems in an attempt to elucidate the carbon dynamics of SPM. SPM was collected from the seagrass ecosystems located at Sikka and Sorong in Indonesia. In situ experiments using SPM traps were conducted to assess the vertical downward flux and decomposition rate of SPM. The isotopic profile of SPM was measured together with organic carbon and total nitrogen content. The results show that SPM was transported to the bottom of the seagrass ecosystem at a rate of up to (129.45±53.79) mg/(m2·h) (according to carbon). Considering the whole period of inundation of seagrass meadows, SPM downward flux reached a maximum of 3 096 mg/(m2·d) (according to carbon). The decomposition rate was estimated at from 5.9 µg/(mg·d) (according to carbon) to 26.6 µg/(mg·d) (according to carbon). Considering the total downward flux of SPM in the study site, the maximum decomposed SPM was estimated 39.9 mg/(m2·d) (according to carbon) and 82.6 mg/(m2·d) (according to carbon) for study site at Sorong and Sikka, respectively. The decomposed SPM can be 0.6%–2.7% of the total SPM flux, indicating that it is a small proportion of the total flux. The seagrass ecosystems of Sorong and Sikka SPM show an autochthonous tendency with the primary composition of marine-end materials.
-
Key words:
- carbon dynamic /
- biogeochemistry /
- coastal ecosystem /
- particulate matter
-
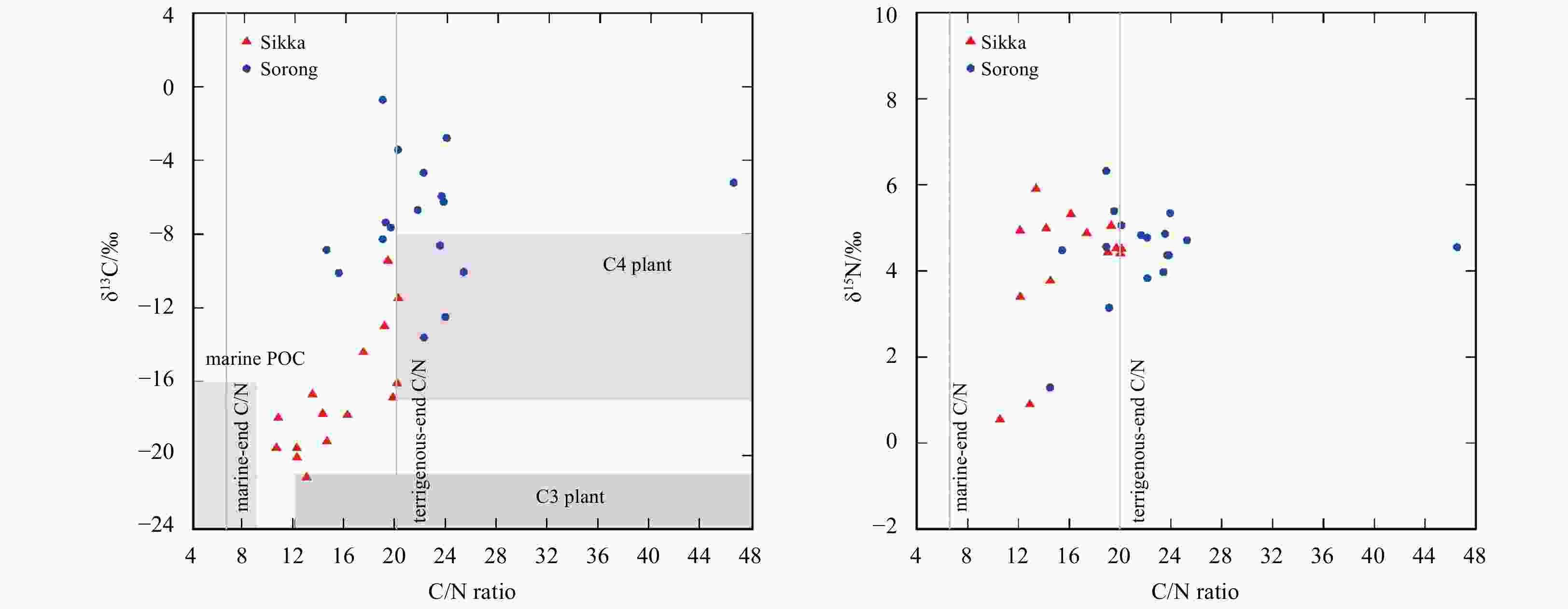
Figure 2. SPM profile according to C/N ratio and isotopic profile of δ13C and δ15N. C/N ratio threshold for marine- and terrigenous-end (6.6 and 20, respectively) follows Martiny et al. (2014) and Gilhooly et al. (2008), respectively. Alternative range of the end-member (shaded area) refers to Lamb et al. (2006). Recorded range of δ13C for seagrasses is from −15.5 to −5.6 (Andrews and Abel, 1979).
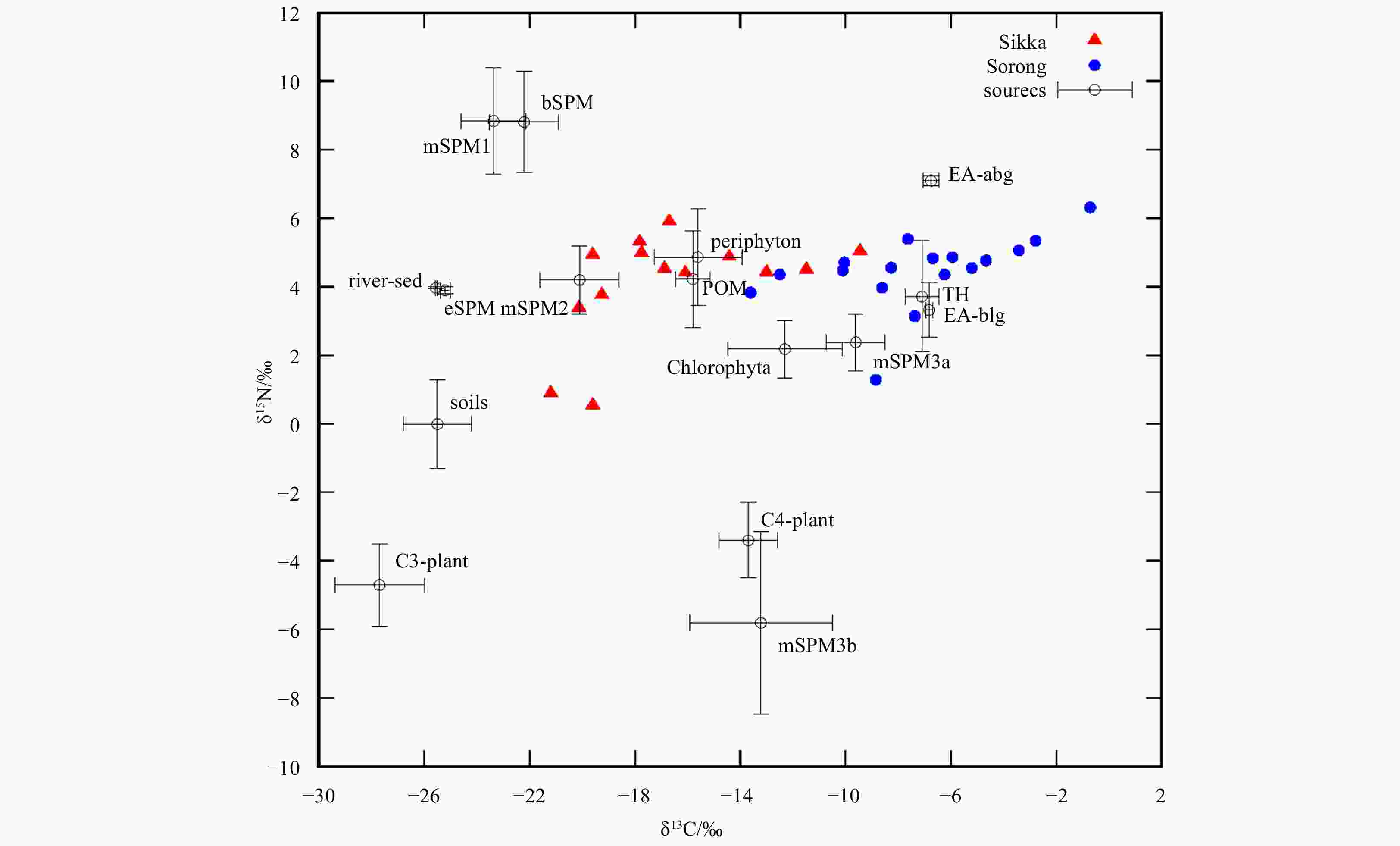
Figure 3. Bi-plot of carbon and nitrogen stable isotopes of SPM to determine the correlation of SPM potential sources. The potential sources refer to Wahyudi and Afdal (2019), namely periphyton, Chlorophyta, C3 and C4 plants, soils, estuary SPM (eSPM), bay SPM (bSPM), river sediment (river-sed), Enhalus acoroides (above and below ground: EA-abg & EA-blg), Thalassia hemprichii (TH), particulate organic matter (POM), and marine SPM (mSPM).
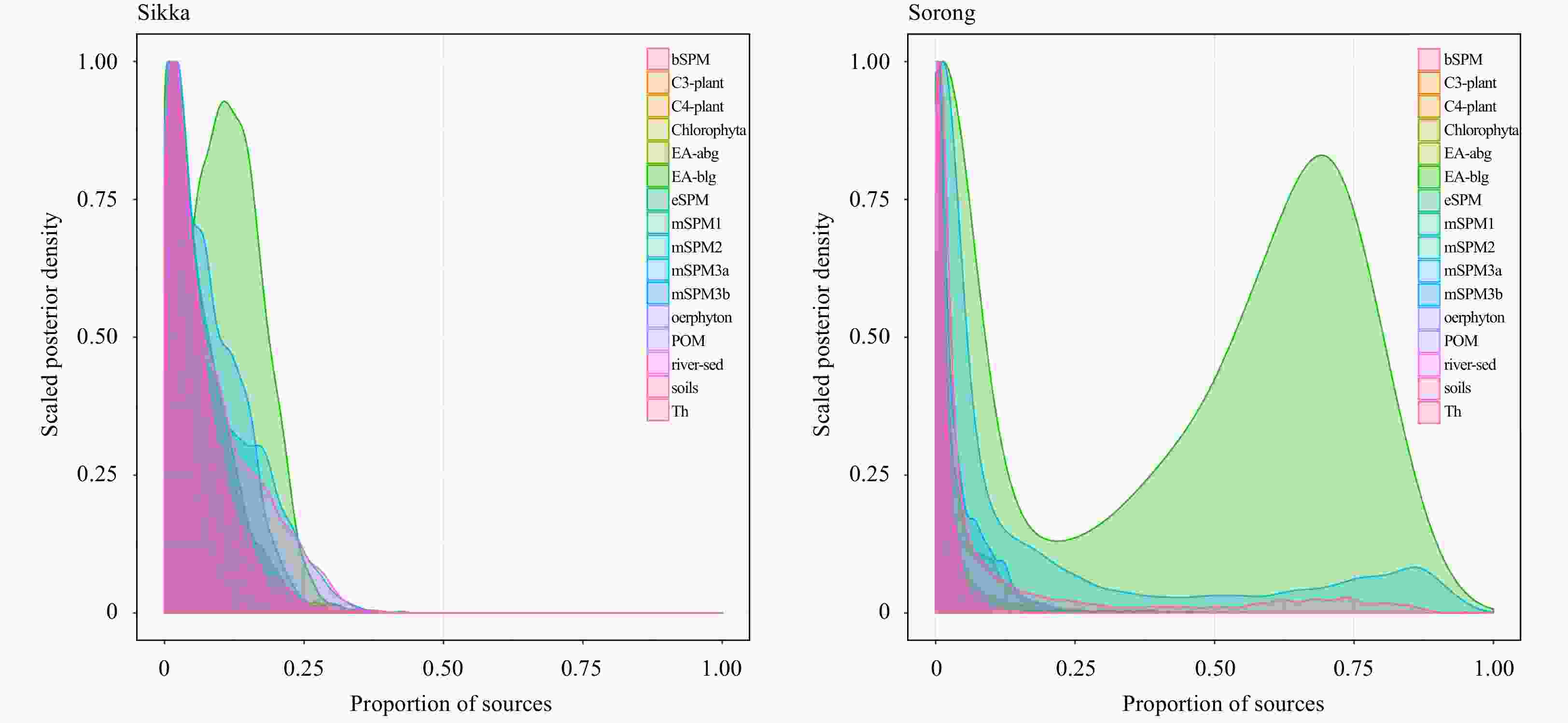
Figure 4. The proportion of sources in the SPM derived from Bayesian mixing model (MixSIAR). The potential sources refer to Wahyudi and Afdal (2019), namely periphyton, Chlorophyta, C3 and C4 plants, soils, estuary SPM (eSPM), bay SPM (bSPM), river sediment (river-sed), Enhalus acoroides (above and below ground: EA-abg & EA-blg), Thalassia hemprichii (Th), particulate organic matter (POM), and marine SPM (mSPM of four locations).
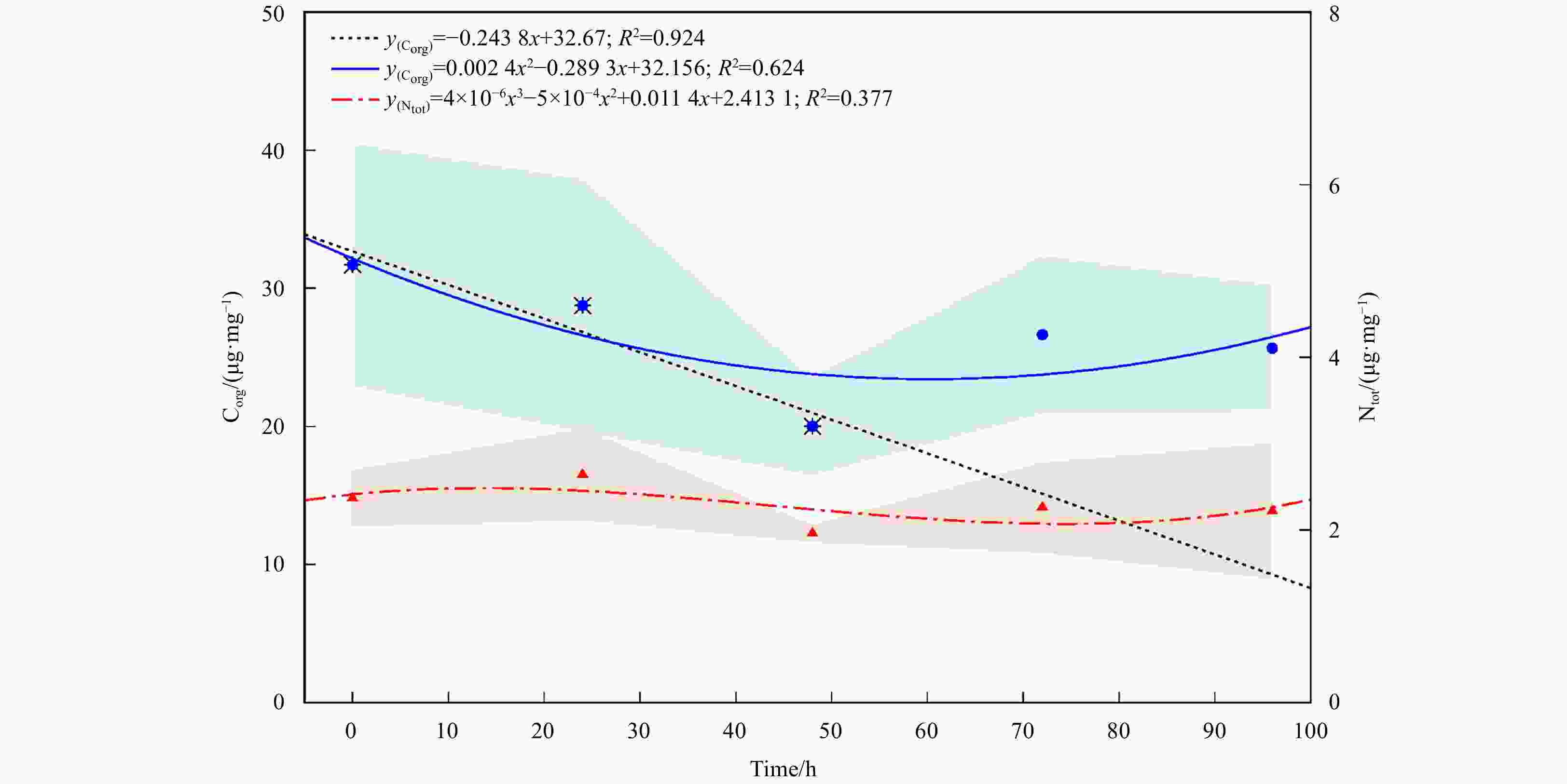
Figure 5. The decomposition rate of SPM from seagrass ecosystems as expressed on organic cabon (Corg) and total nitrogen (Ntot). Black dashed line (and ӿ symbol) shows the linear regression of decomposition rate of SPM’s organic carbon for the 48 h; blue line (and blue filled circle) shows the polynomial regression of organic carbon decomposition rate for the whole experiment periods; red dashed line (and red filled triangle) shows decomposition rate of SPM’s total nitrogen. Shaded area shows the standard deviation.
Table 1. SPM isotopic profile and vertical flux of seagrass ecosystems in the Sikka and Sorong notated in average ± Std Dev
Location δ15N
/‰δ13C
/‰C:N ratio Ntot flux
/(µg·h−1)*Corg flux
/(µg·h−1)*Ntot flux
/(mg·m−2·h−1)
(according to nitrogen)Corg flux
/(mg·m−2·h−1)
(according to carbon)Sikka 4.10±1.56 −16.76±3.36 15.47±3.53 2.96±0.72 38.48±10.10 4.80±1.17 62.52±16.41 Sorong 4.01±1.36 −7.22±3.36 22.50±6.89 5.89±3.41 79.67±33.10 9.56±5.54 129.45±53.79 Note: * Hourly flux of Corg and Ntot trapped in the SPM trap (mouth area: 6.154 4 cm2). -
[1] Andrews T J, Abel K M. 1979. Photosynthetic carbon metabolism in seagrasses 14C-labeling evidence for the C3 pathway. Plant Physiology, 63(4): 650–656. doi: 10.1104/pp.63.4.650 [2] Andrews J E, Greenaway A M, Dennis P F. 1998. Combined carbon isotope and C/N ratios as indicators of source and fate of organic matter in a poorly flushed, tropical estuary: Hunts Bay, Kingston Harbour, Jamaica. Estuarine, Coastal and Shelf Science, 46(5): 743–756. doi: 10.1006/ecss.1997.0305 [3] Anthony K R N. 1999. Coral suspension feeding on fine particulate matter. Journal of Experimental Marine Biology and Ecology, 232(1): 85–106. doi: 10.1016/S0022-0981(98)00099-9 [4] Bao Hongyan, Wu Ying, Tian Lixin, et al. 2013. Sources and distributions of terrigenous organic matter in a mangrove fringed small tropical estuary in South China. Acta Oceanologica Sinica, 32(4): 18–26. doi: 10.1007/s13131-013-0295-3 [5] Bar-Zeev E, Berman-Frank I, Stambler N, et al. 2009. Transparent exopolymer particles (TEP) link phytoplankton and bacterial production in the Gulf of Aqaba. Aquatic Microbial Ecology, 56(2–3): 217–225. doi: 10.3354/ame01322 [6] Bouillon S, Connolly R M. 2009. Carbon exchange among tropical coastal ecosystems. In: Nagelkerken I, ed. Ecological Connectivity among Tropical Coastal Ecosystems. Dordrecht, the Netherlands: Springer, 45–70, doi: 10.1007/978-90-481-2406-0_3 [7] Dalu T, Richoux N B, Froneman P W. 2016. Nature and source of suspended particulate matter and detritus along an austral temperate river–estuary continuum, assessed using stable isotope analysis. Hydrobiologia, 767(1): 95–110. doi: 10.1007/s10750-015-2480-1 [8] Dehwah A H A, Anderson D M, Li Sheng, et al. 2019. Transparent exopolymer particle binding of organic and inorganic particles in the Red Sea: implications for downward transport of biogenic materials. Biogeosciences Discussions, 1–40. doi: 10.5194/bg-2019-59 [9] Duarte C M, Kennedy H, Marbà N, et al. 2013. Assessing the capacity of seagrass meadows for carbon burial: current limitations and future strategies. Ocean & Coastal Management, 83: 32–38. doi: 10.1016/j.ocecoaman.2011.09.001 [10] Fernandes L, Bhosle N B, Matondkar S G P, et al. 2009. Seasonal and spatial distribution of particulate organic matter in the Bay of Bengal. Journal of Marine Systems, 77(1–2): 137–147. doi: 10.1016/j.jmarsys.2008.12.002 [11] Fettweis M, Baeye M. 2015. Seasonal variation in concentration, size, and settling velocity of muddy marine flocs in the benthic boundary layer. Journal of Geophysical Research: Oceans, 120(8): 5648–5667. doi: 10.1002/2014JC010644 [12] Gasparini S, Castel J, Irigoien X. 1999. Impact of suspended particulate matter on egg production of the estuarine copepod, Eurytemora affinis. Journal of Marine Systems, 22(2–3): 195–205. doi: 10.1016/S0924-7963(99)00041-X [13] Gilhooly III W P, Macko S A, Flemings P B. 2008. Data report: isotope compositions of sedimentary organic carbon and total nitrogen from Brazos-Trinity Basin IV (Sites U1319 and U1320) and Ursa Basin (Sites U1322 and U1324), deepwater Gulf of Mexico. In: Flemings P B, Behrmann J H, John C M, et al, eds. Proceedings of the International Ocean Discovery Program. College Station, TX, USA: Integrated Ocean Drilling Program Management International, Inc., doi: 10.2204/iodp.proc.308.208.2008 [14] Harrison P G. 1989. Detrital processing in seagrass systems: a review of factors affecting decay rates, remineralization and detritivory. Aquatic Botany, 35(3–4): 263–288. doi: 10.1016/0304-3770(89)90002-8 [15] Hedges J I, Baldock J A, Gélinas Y, et al. 2001. Evidence for non-selective preservation of organic matter in sinking marine particles. Nature, 409(6822): 801–804. doi: 10.1038/35057247 [16] Holmer M, Olsen A B. 2002. Role of decomposition of mangrove and seagrass detritus in sediment carbon and nitrogen cycling in a tropical mangrove forest. Marine Ecology Progress Series, 230: 87–101 [17] Kaiser D, Unger D, Qiu Guanglong. 2014. Particulate organic matter dynamics in coastal systems of the northern Beibu Gulf. Continental Shelf Research, 82: 99–118. doi: 10.1016/j.csr.2014.04.006 [18] Karl D M, Knauer G A, Martin J H. 1988. Downward flux of particulate organic matter in the ocean: a particle decomposition paradox. Nature, 332(6163): 438–441. doi: 10.1038/332438a0 [19] Kennedy H, Beggins J, Duarte C M, et al. 2010. Seagrass sediments as a global carbon sink: isotopic constraints. Global Biogeochemical Cycles, 24(4): GB4026. doi: 10.1029/2010GB003848 [20] Kennedy H, Gacia E, Kennedy D P, et al. 2004. Organic carbon sources to SE Asian coastal sediments. Estuarine, Coastal and Shelf Science, 60(1): 59–68. doi: 10.1016/j.ecss.2003.11.019 [21] Lamb A L, Wilson G P, Leng M J. 2006. A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. Earth-Science Reviews, 75(1–4): 29–57. doi: 10.1016/j.earscirev.2005.10.003 [22] Lavery P S, McMahon K, Weyers J, et al. 2013. Release of dissolved organic carbon from seagrass wrack and its implications for trophic connectivity. Marine Ecology Progress Series, 494: 121–133. doi: 10.3354/meps10554 [23] Liu K K, Kao S J, Hu H C, et al. 2007a. Carbon isotopic composition of suspended and sinking particulate organic matter in the northern South China Sea—from production to deposition. Deep-Sea Research Part II: Topical Studies in Oceanography, 54(14–15): 1504–1527. doi: 10.1016/j.dsr2.2007.05.010 [24] Liu K K, Kao S J, Wen L S, et al. 2007b. Carbon and nitrogen isotopic compositions of particulate organic matter and biogeochemical processes in the eutrophic Danshuei Estuary in northern Taiwan. Science of the Total Environment, 382(1): 103–120. doi: 10.1016/j.scitotenv.2007.04.019 [25] Martiny A C, Vrugt J A, Lomas M W. 2014. Concentrations and ratios of particulate organic carbon, nitrogen, and phosphorus in the global ocean. Scientific Data, 1: 140048. doi: 10.1038/sdata.2014.48 [26] McTigue N D, Dunton K H. 2014. Trophodynamics and organic matter assimilation pathways in the northeast Chukchi Sea, Alaska. Deep-Sea Research Part II: Topical Studies in Oceanography, 102: 84–96. doi: 10.1016/j.dsr2.2013.07.016 [27] Middelburg J J, Herman P M J. 2007. Organic matter processing in tidal estuaries. Marine Chemistry, 106(1–2): 127–147. doi: 10.1016/j.marchem.2006.02.007 [28] Mills M M, Lipschultz F, Sebens K P. 2004. Particulate matter ingestion and associated nitrogen uptake by four species of scleractinian corals. Coral Reefs, 23(3): 311–323. doi: 10.1007/s00338-004-0380-3 [29] Narulita I, Rahayu R, Kusratmoko E, et al. 2019. Ancaman kekeringan meteorologis di pulau Kecil tropis akibat pengaruh El-Nino Dan Indian Ocean dipole (IOD) positif, studi kasus: Pulau Bintan. Jurnal Lingkungan dan Bencana Geologi, 10(3): 127–138. doi: 10.34126/JLBG.V10I3.252 [30] Post D M. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology, 83(3): 703–718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2 [31] Prasad M B K, Ramanathan A L. 2009. Organic matter characterization in a tropical estuarine-mangrove ecosystem of India: preliminary assessment by using stable isotopes and lignin phenols. Estuarine, Coastal and Shelf Science, 84(4): 617–624. doi: 10.1016/j.ecss.2009.07.029 [32] Saavedra-Hortua D A, Friess D A, Zimmer M, et al. 2020. Sources of particulate organic matter across mangrove forests and adjacent ecosystems in different geomorphic settings. Wetlands, 40(5): 1047–1059. doi: 10.1007/s13157-019-01261-9 [33] Sachoemar S I, Yanagi T. 2001. Seasonal variation of the oceanic condition along the southern coastal area of Java to Sumbawa, Indonesia. La mer, 39(3): 141–154 [34] Schlacher T A, Connolly R M. 2014. Effects of acid treatment on carbon and nitrogen stable isotope ratios in ecological samples: a review and synthesis. Methods in Ecology and Evolution, 5(6): 541–550. doi: 10.1111/2041-210X.12183 [35] Seiki T, Date E, Izawa H. 1991. Decomposition characteristics of particulate organic matter in Hiroshima Bay. Journal of the Oceanographical Society of Japan, 47(5): 207–220. doi: 10.1007/BF02310036 [36] Simon M, Grossart H P, Schweitzer B, Ploug H. 2002. Microbial ecology of organic aggregates in aquatic ecosystems. Aquatic Microbial Ecology, 28(2): 175–211. doi: 10.3354/ame028175 [37] Sjafrie N D M, Hernawan U E, Rahmawati S, et al. 2018. Status of the seagrass meadows in Indonesia 2018. Jakarta, Indonesia: Indonesian Institute of Sciences, https://marxiv.org/juk4h/[2020-1-20] [38] Sobczak W V, Cloern J E, Jassby A D, et al. 2002. Bioavailability of organic matter in a highly disturbed Estuary: the role of detrital and algal resources. Proceedings of the National Academy of Sciences of the United States of America, 99(12): 8101–8105. doi: 10.1073/pnas.122614399 [39] Stock B, Semmens B. 2016. MixSIAR GUI user manual. Version 3.1, doi: 10.5281/zenodo.47719 [40] Touchette B W, Burkholder J M. 2000. Overview of the physiological ecology of carbon metabolism in seagrasses. Journal of Experimental Marine Biology and Ecology, 250(1–2): 169–205. doi: 10.1016/S0022-0981(00)00196-9 [41] Volkman J K, Revill A T, Holdsworth D G, et al. 2008. Organic matter sources in an enclosed coastal inlet assessed using lipid biomarkers and stable isotopes. Organic Geochemistry, 39(6): 689–710. doi: 10.1016/j.orggeochem.2008.02.014 [42] Wahyudi A J, Afdal A. 2019. The origin of the suspended particulate matter in the seagrass meadow of tropical waters, an evidence of the stable isotope signatures. Acta Oceanologica Sinica, 38(1): 136–143. doi: 10.1007/s13131-019-1380-z [43] Wahyudi A J, Meirinawati H, Prayitno H B, et al. 2019. The material origin of the particulate organic matter (POM) in the Eastern Indonesian waters. AIP Conference Proceedings, 2175(1): 020047. doi: 10.1063/1.5134611 [44] Wahyudi A J, Rahmawati S, Irawan A, et al. 2020. Assessing carbon stock and sequestration of the tropical seagrass meadows in Indonesia. Ocean Science Journal, 55(1): 85–97. doi: 10.1007/s12601-020-0003-0 [45] Wahyudi A J, Rahmawati S, Prayudha B, et al. 2016. Vertical carbon flux of marine snow in Enhalus acoroides-dominated seagrass meadows. Regional Studies in Marine Science, 5: 27–34. doi: 10.1016/j.rsma.2016.01.003 [46] Wahyudi A J, Wada S, Aoki M, et al. 2013. Stable isotope signature and pigment biomarker evidence of the diet sources of Gaetice depressus (Crustacea: Eubrachyura: Varunidae) in a boulder shore ecosystem. Plankton and Benthos Research, 8(2): 55–67. doi: 10.3800/pbr.8.55 [47] Zhang J, Wu Yadian, Jennerjahn T C, et al. 2007. Distribution of organic matter in the Changjiang (Yangtze River) Estuary and their stable carbon and nitrogen isotopic ratios: implications for source discrimination and sedimentary dynamics. Marine Chemistry, 106(1–2): 111–126. doi: 10.1016/j.marchem.2007.02.003 -




 下载:
下载: