Changes of melatonin and its receptors in synchronizing turbot (Scophthalmus maximus) seasonal reproduction and maturation rhythm
-
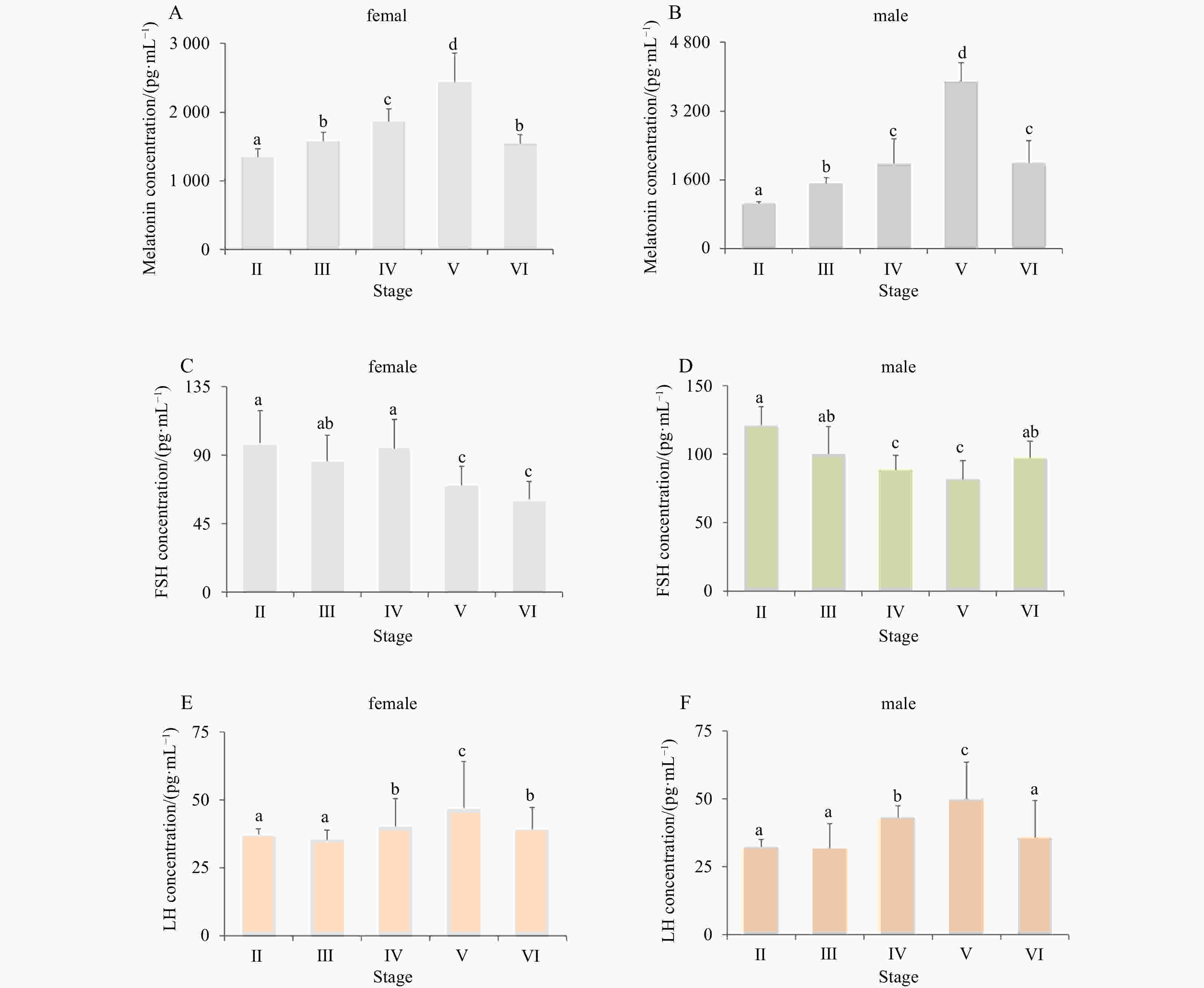
Abstract: In most fish, reproduction is seasonal or periodic under the suitable conditions. In turbot (Scophthalmus maximus) farms, one of the most economically important marine flatfish species, changes in daylength could cause changes in the spawning time. In this study, to characterize the regulation of reproductive physiology following light signals, three melatonin receptors (Mtnr) investigated in turbot were named smMtnr1, smMtnr2, and smMtnr1c. Distinct expression profiles demonstrated that Mtnr mRNAs were concentrated in the brain (as detected in the hypothalamus (Hy) and mesencephalon (Me)), gonad and eye. The most abundant Mtnr1 and Mtnr2 mRNA expression levels were detected in the central nervous system at the beginning of the breeding season, suggesting that Mtnr1 and Mtnr2 may play vital roles in the regulation of turbot gonadal development. In addition, the melatonin profiles gradually increased and reached to the highest level at the spawning stage, indicating that melatonin is a potent hormone in the regulation of fish oocyte growth and maturation. The results of this study suggested that melatonin is the primary factor that transduces the light signal and regulates the physiological functions of turbot seasonal reproduction. Moreover, the results of this study may establish a foundation for further research seeking to identify fish melatonin receptors involved in the gonadal development and gamete maturation.
-
Key words:
- turbot /
- brain /
- melatonin /
- melatonin receptors /
- seasonal reproductive development
-
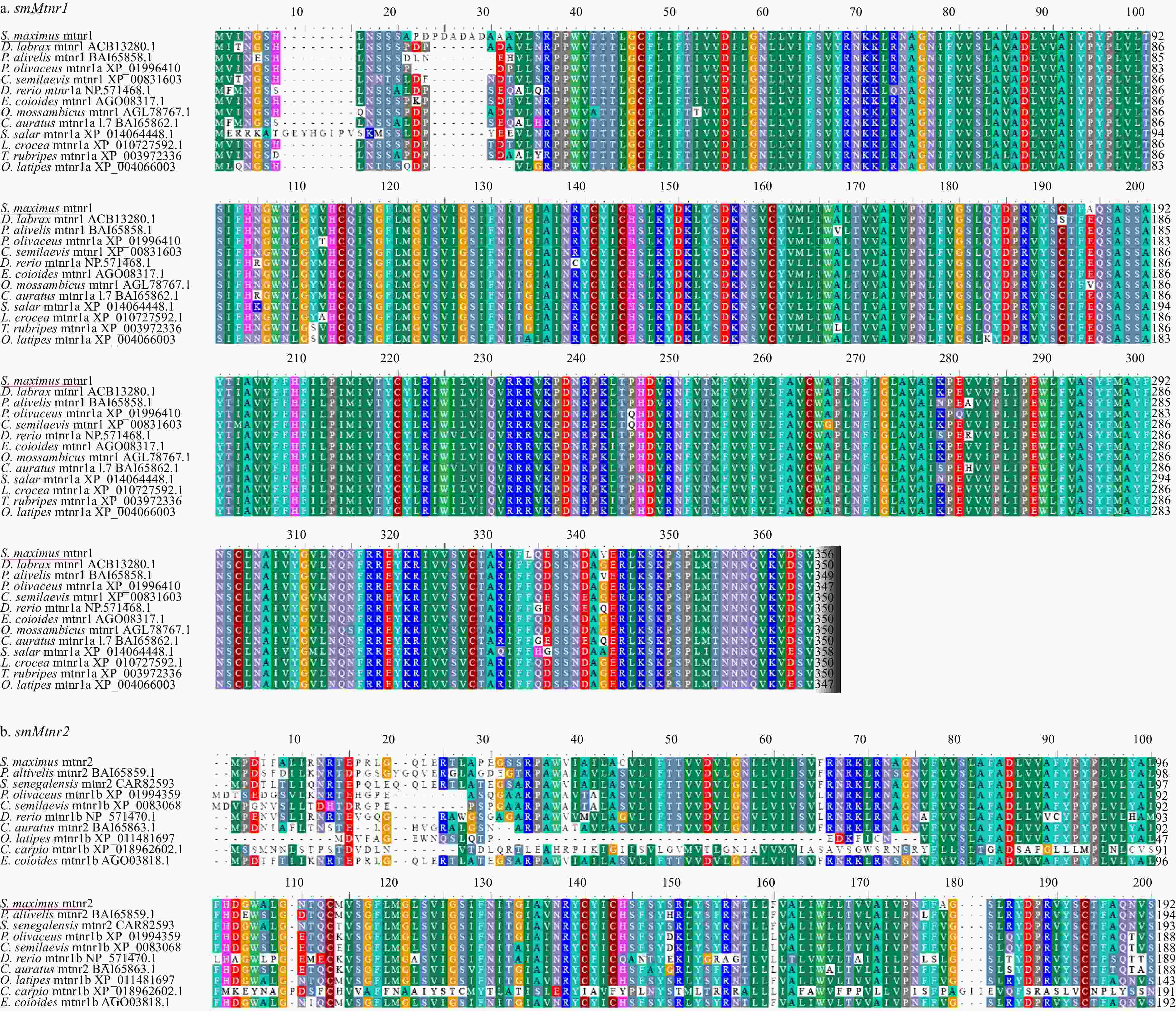
Figure 3. Phylogenetic tree of deduced amino acid sequences for three smMtnrs from turbot and other vertebrates was constructed by Mega7 with neighbor-joining method. The numbers adjacent to nodes indicate bootstrap percentage value for 1000 replicates (>80%). The GenBank accession numbers of the sequences presented before the species name; and smMtnr1, smMtnr2, and smMtnr1c cDNAs are with the GenBank accession numbers of MK738109, MK738110 and MK738111.
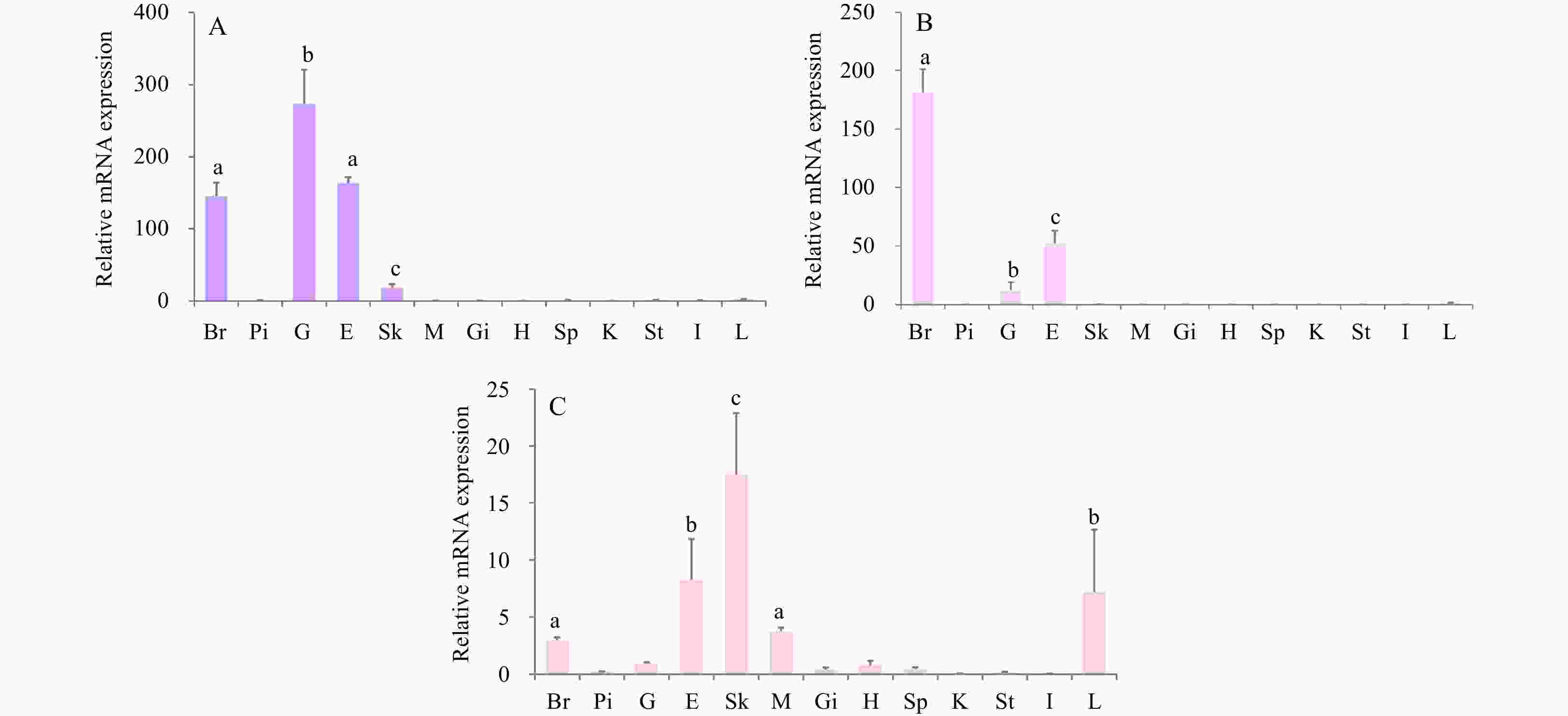
Figure 4. The tissue distribution of three smMtnr genes in turbot. A. Expression of smMtnr1; B. expression of smMtnr2; C. expression of smMtnr1c. The relative abundance of three smMtnr genes were expressed as mean ± SEM (n=3). Tissue abbreviations: Br, brain; Pi, pituitary; G, gonad; E, eye; Sk, skin; M, muscle; Gi, gill; H, heart; Sp, spleen; K, kidney; St, stomach; I, intestine; L, liver. Different letters above bars represent statistical significant (p < 0.05).
Figure 5. Expression of three smMtnr genes in different brain areas and eye. (A) Expression of smMtnr1; (B) expression of smMtnr2; (C) expression of smMtnr1c; (D) dissected brain areas used for expression studies. Ey, eye; Ob, olfactory bulbs; Te, telencephalon; Me, mesencephalon; Ce, cerebellum; Hy, hypothalamus; Pi, pituitary gland; Mo, medulla oblongata. Different letters above bars represent statistical significant (p < 0.05).
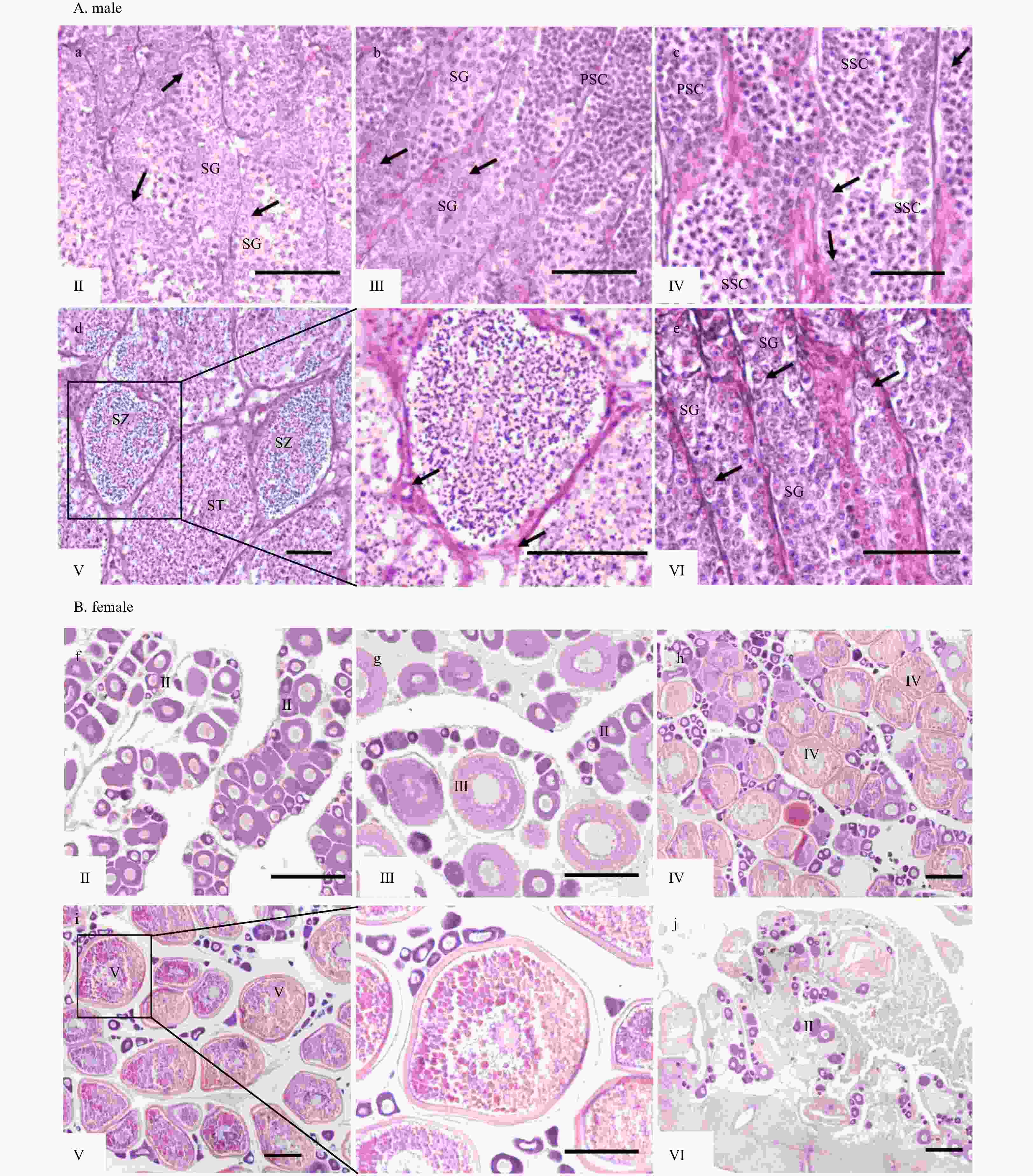
Figure 6. Histology of testes and ovaries during reproductive cycle. Tissue abbreviations: PSC, primary spermatocytes; SG, spermatogonia; SSC, secondary spermatocytes; ST, spermatids; SZ, spermatozoa; I, previtellogenic oocytes; II, primary vitellogenic oocytes; III−IV, large growth of vitellogenic oocytes. Scale: A (a−e), 50 μm; B (f−j), 200 μm.
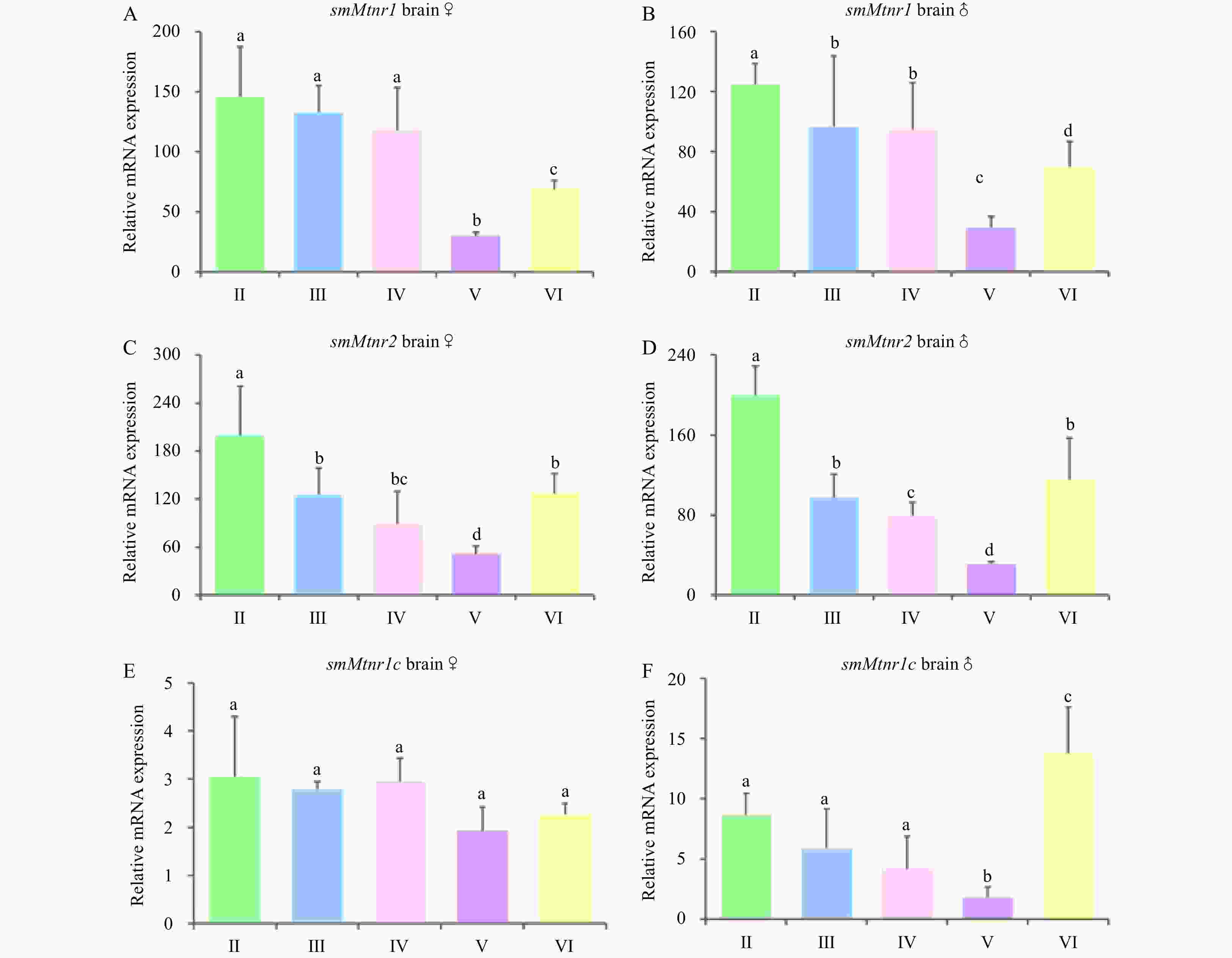
Figure 7. Expression of three smMtnr genes throughout the reproductive cycle in turbot brain of male and female. Error bars are presented as the mean±SEM. Different letters above bars represent statistical significance (p < 0.05) between two sexual maturation stages. The internal control was β-actin.
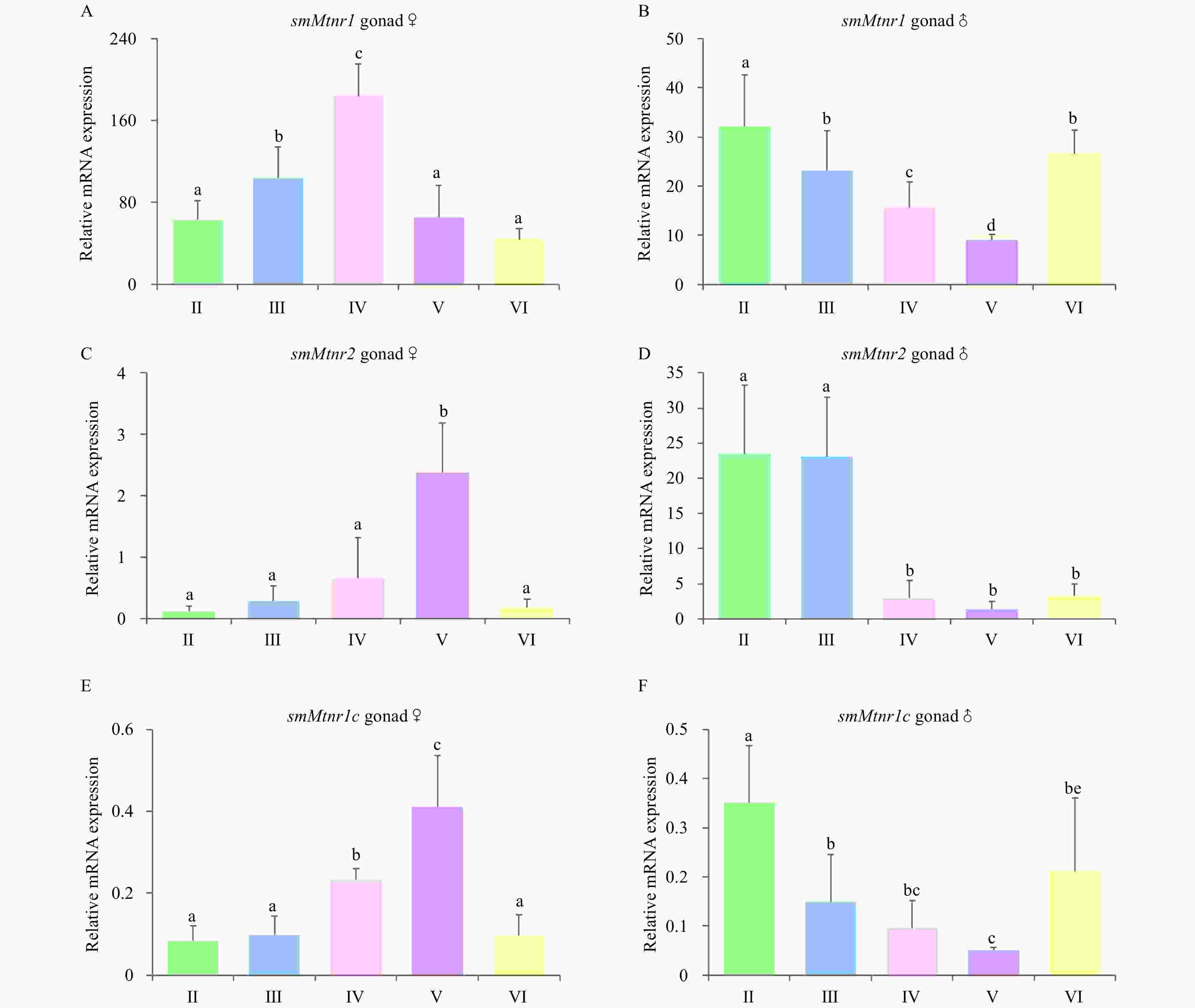
Figure 8. Expression of three smMtnr genes throughout the reproductive cycle in turbot gonad of male and female. Error bars are presented as the mean±SEM. Different letters above bars represent statistical significance (p < 0.05) between two sexual maturation stages. The internal control was β-actin.
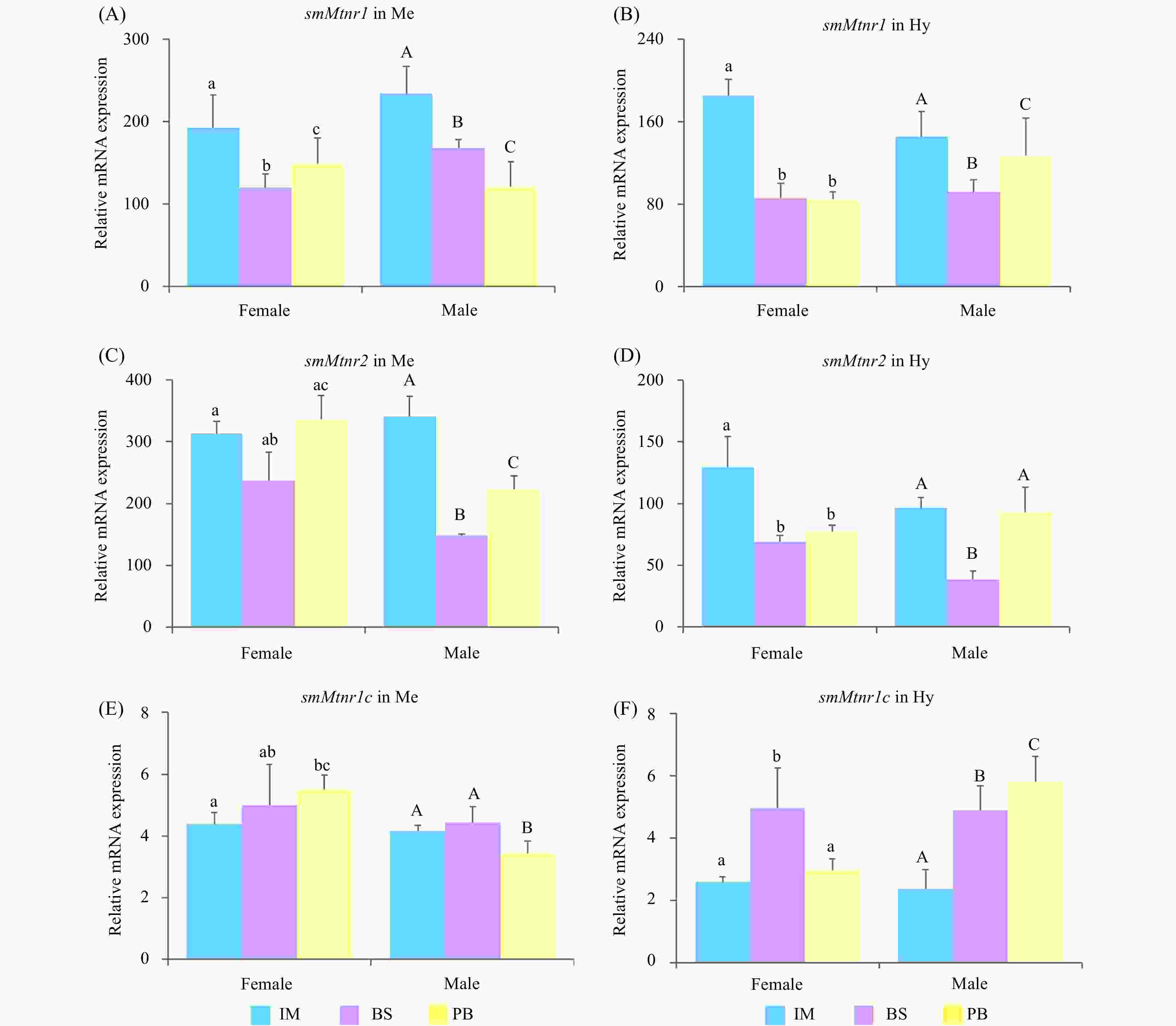
Figure 9. Expression of three smMtnr genes in mesencephalon (Me) and hypothalamus (Hy) in immature stage (IM), the breeding season (BS) and post-breeding season (PB). Error bars are presented as the mean±SEM. Different letters above bars represent statistical significance (p < 0.05). The internal control was β-actin.
Table 1. List of primers used for molecular cloning of Mtnr cDNAs and qPCR
Primer name Nucleotide sequence (5'-3') Purpose/Products Mtnr1-F AAATGGGTCTCACCTGAACAGC fragment PCR (759 bp) Mtnr1-R AAACGGCGAAGAGCACAAA Mtnr2-F ACTGCTACATCTGTCACTCG fragment PCR (480 bp) Mtnr2-R AGTAGGCCATGAAGTAGCTG Mtnr1c-F CACTTTCTTATCCCGCTTCT fragment PCR (424 bp) Mtnr1c-R GGCTTACTCTTCAGTCCCTC 5'- Mtnr1-GSP1 CCCAGCAAACGGCGAAGAGCACAAA 5' RACE-PCR 5'- Mtnr1-GSP2 GACCAGCAGGTTGCCCAGGATGTCG 5' RACE-PCR (nested) 3'- Mtnr1-GSP1 TCTGCCACAGCCTCAAATATGATAAAC 3' RACE-PCR 3'- Mtnr1-GSP2 GTTTGTGCTCTTCGCCGTTTGCT 3' RACE-PCR (nested) 5'- Mtnr2-GSP1 CTCTCCTCGGTCTTCACTTTACG 5' RACE-PCR 5'- Mtnr2-GSP2 AGGTCAGCGAACGCCAAACTCAC 5' RACE-PCR (nested) 3'- Mtnr2-GSP1 CGCTGACCTCGTGGTAGCCTTCT 3' RACE-PCR 3'- Mtnr2-GSP2 GAGCCCTCGCCTCCGACCAAGTG 3' RACE-PCR (nested) 5'- Mtnr1c-GSP1 AAGAGCGAGGAGGATCGTCTTGTAC 5' RACE-PCR 5'- Mtnr1c-GSP2 CCTCAGGCTGAACAAACGGTCGTAG 5' RACE-PCR (nested) 3'- Mtnr1c-GSP1 GAAAGTGGCGCCCCACATTCCTGAGTG 3' RACE-PCR 3'- Mtnr1c-GSP2 GTAGCGGAGATGAATGTATGAACAACTG 3' RACE-PCR (nested) sm- Mtnr1-F AACCTGGGTTACGTCCACTG 224 bp sm- Mtnr1-R AGCGAACCCACAAAGAGGTT sm- Mtnr2-F CGTGGTCTTTGTGCTGTTCG 204 bp sm- Mtnr2-R ATGCGCTTGTACTCGTTCCT sm- Mtnr1c-F CAAGACGATCCTCCTCGCTC 185 bp sm- Mtnr1c-R GGCGAGGCTCCAGATAACAA sm-β-actin-F GCTGTGCTGTCCCTGTATGCC 187 bp sm-β-actin-R AGGAGTAGCCACGCTCTGTCA Table 2. Characteristics of Mtnrs in turbot
Gene Chr CDS pI MWs Transmembrane Transmembrane regions Mtnr1 8 357 8.90 40001.32 7 TM 36−58, 70−92, 106−129, 149−172, 193−214, 247−269, 282−302 Mtnr2 4 379 9.32 42846.41 7 TM 33−55, 72−98, 109−131, 152−174, 199−221, 248−270, 285−308 Mtnr1c 13 362 8.65 40211.51 7 TM 42−64, 76−98, 113−137, 155−178, 203−225, 246−267, 288−310 Note: CDS, coding sequence; Chr, chromosome; pI, isoelectric point; MWs, molecular weights. -
[1] Alvariño J M R, Rebollar P G, Olmedo M, et al. 2001. Effects of melatonin implants on reproduction and growth of turbot broodstock. Aquaculture International, 9(6): 477–487 [2] Boeuf G, Le Bail P Y. 1999. Does light have an influence on fish growth?. Aquaculture, 177(1–4): 129–152 [3] Carnevali O, Gioacchini G, Piccinetti C C, et al. 2010. Melatonin control of oogenesis and metabolic resources in Zebrafish. Journal of Applied Ichthyology, 26(5): 826–830 [4] Chai Ke, Liu Xiaochun, Zhang Yong, et al. 2013. Day-night and reproductive cycle profiles of melatonin receptor, kiss, and gnrh expression in orange-spotted grouper (Epinephelus coioides). Molecular Reproduction and Development, 80(7): 535–548 [5] Chattoraj A, Seth M, Basu A, et al. 2009. Temporal relationship between the circulating profiles of melatonin and ovarian steroids under natural photo-thermal conditions in an annual reproductive cycle in carp Catla catla. Biological Rhythm Research, 40(4): 347–359 [6] Choi C Y, Shin H S, Kim N N, et al. 2015. Time-related effects of various LED light spectra on reproductive hormones in the brain of the goldfish Carassius auratus. Biological Rhythm Research, 46(5): 671–682 [7] Ciani E, Fontaine R, Maugars G, et al. 2019. Melatonin receptors in Atlantic salmon stimulate cAMP levels in heterologous cell lines and show season-dependent daily variations in pituitary expression levels. Journal of Pineal Research, 67(3): e12590 [8] Confente F, Rendón M C, Besseau L, et al. 2010. Melatonin receptors in a pleuronectiform species, Solea senegalensis: Cloning, tissue expression, day–night and seasonal variations. General and Comparative Endocrinology, 167(2): 202–214 [9] Coon S L, Klein D C. 2006. Evolution of arylalkylamine N-acetyltransferase: emergence and divergence. Molecular and Cellular Endocrinology, 252(1–2): 2–10 [10] Danilova N, Krupnik V E, Sugden D, et al. 2004. Melatonin stimulates cell proliferation in zebrafish embryo and accelerates its development. The FASEB Journal, 18(6): 751–753 [11] Davies B, Hannah L T, Randall C F, et al. 1994. Central melatonin binding sites in rainbow trout (Onchorhynchus mykiss). General and Comparative Endocrinology, 96(1): 19–26 [12] Elakkanai P, Francis T, Ahilan B, et al. 2015. Role of GnRH, HCG and Kisspeptin on reproduction of fishes. Indian Journal of Science and Technology, 8(17): 65166 [13] Falcón J, Besseau L, Sauzet S, et al. 2007. Melatonin effects on the hypothalamo-pituitary axis in fish. Trends in Endocrinology & Metabolism, 18(2): 81–88 [14] Falcón J, Migaud H, Munoz-Cueto J A, et al. 2010. Current knowledge on the melatonin system in teleost fish. General and Comparative Endocrinology, 165(3): 469–482 [15] Forés R, Iglesias J, Olmedo M, et al. 1990. Induction of spawning in turbot (scophthalmus maximus L. ) by a sudden change in the photoperiod. Aquacultural Engineering, 9(5): 357–366 [16] Forsell J, Holmqvist B O, Helvik J V, et al. 1997. Role of the pineal organ in the photoregulated hatching of the Atlantic halibut. International Journal of Developmental Biology, 41(4): 591–595 [17] Gaeta L M, Tozzi G, Pastore A, et al. 2002. Determination of superoxide dismutase and glutathione peroxidase activities in blood of healthy pediatric subjects. Clinica Chimica Acta, 322(1–2): 117–120 [18] Galano A, Tan D X, Reiter R J. 2013. On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. Journal of Pineal Research, 54(3): 245–257 [19] Gopurappilly R, Ogawa S, Parhar I S. 2013. Functional significance of GnRH and kisspeptin, and their cognate receptors in teleost reproduction. Frontiers in Endocrinology, 4: 24 [20] Hastings M H, Walker A P, Herbert J. 1987. Effect of asymmetrical reductions of photoperiod on pineal melatonin, locomotor activity and gonadal condition of male syrian hamsters. Journal of Endocrinology, 114(2): 221–229 [21] Hong Luyan, Hong Wanshu, Zhu Wenbo, et al. 2014. Cloning and expression of melatonin receptors in the mudskipper Boleophthalmus pectinirostris: their role in synchronizing its semilunar spawning rhythm. General and Comparative Endocrinology, 195: 138–150 [22] Ikegami T, Azuma K, Nakamura M, et al. 2009. Diurnal expressions of four subtypes of melatonin receptor genes in the optic tectum and retina of goldfish. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 152(2): 219–224 [23] Ikegami K, Yoshimura T. 2016. Comparative analysis reveals the underlying mechanism of vertebrate seasonal reproduction. General and Comparative Endocrinology, 227: 64–68 [24] Imsland A K, Gunnarsson S, Roth B, et al. 2013. Long-term effect of photoperiod manipulation on growth, maturation and flesh quality in turbot. Aquaculture, 416–417: 152–160 [25] Kim B H, Hur S P, Hyeon J Y, et al. 2020. Annual patterns of ocular melatonin level in the female grass puffer, Takifugu alboplumbeus: possible involvement in seasonal reproductive response. Fish Physiology and Biochemistry, 46(3): 787–801 [26] Kim J H, Park J W, Kwon J Y. 2018. Effects of exogenous melatonin on the reproductive activities of Nile tilapia, Oreochromis niloticus. Biological Rhythm Research, 49(3): 392–404 [27] Kochman K. 2012. Evolution of gonadotropin-releasing hormone (GnRH) structure and its receptor. Journal of Animal and Feed Sciences, 21(1): 3–30 [28] Kulczykowska E, Kalamarz H, Warne J M, et al. 2006. Day-night specific binding of 2-[125I] iodomelatonin and melatonin content in gill, small intestine and kidney of three fish species. Journal of Comparative Physiology B, 176(4): 277–285 [29] Lan-Chow-Wing O, Confente F, Herrera-Pérez P, et al. 2014. Distinct expression profiles of three melatonin receptors during early development and metamorphosis in the flatfish Solea senegalensis. International Journal of Molecular Sciences, 15(11): 20789–20799 [30] Maitra S K, Chattoraj A, Mukherjee S, et al. 2013. Melatonin: A potent candidate in the regulation of fish oocyte growth and maturation. General and Comparative Endocrinology, 181: 215–222 [31] Maitra S K, Hasan K N. 2016. The role of melatonin as a hormone and an antioxidant in the control of fish reproduction. Frontiers in Endocrinology, 7: 38 [32] Mazurais D, Brierley I, Anglade I, et al. 1999. Central melatonin receptors in the rainbow trout: Comparative distribution of ligand binding and gene expression. Journal of Comparative Neurology, 409(2): 313–324 [33] Moniruzzaman M, Hasan K N, Maitra S K. 2016. Melatonin actions on ovaprim (synthetic GnRH and domperidone)—induced oocyte maturation in carp. Reproduction, 151(4): 285–296 [34] Moniruzzaman M, Maitra S K. 2012. Influence of altered photoperiods on serum melatonin and its receptors (MT1 and MT2) in the brain, retina, and ovary in carp Catla catla. Chronobiology International, 29(2): 175–188 [35] Nishiwaki-Ohkawa T, Yoshimura T. 2016. Molecular basis for regulating seasonal reproduction in vertebrates. Journal of Endocrinology, 229(3): R117–R127 [36] Park Y J, Park J G, Hiyakawa N, et al. 2007a. Diurnal and circadian regulation of a melatonin receptor, MT1, in the golden rabbitfish, Siganus guttatus. General and Comparative Endocrinology, 150(2): 253–262 [37] Park Y J, Park J G, Jeong H B, et al. 2007b. Expression of the melatonin receptor Mel1c in neural tissues of the reef fish Siganus guttatus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 147(1): 103–111 [38] Park Y J, Park J G, Kim S J, et al. 2006. Melatonin receptor of a reef fish with lunar-related rhythmicity: cloning and daily variations. Journal of Pineal Research, 41(2): 166–174 [39] Patiño M A L, Alonso-Gómez A L, Guijarro A, et al. 2008. Melatonin receptors in brain areas and ocular tissues of the teleost Tinca tinca: Characterization and effect of temperature. General and Comparative Endocrinology, 155(3): 847–856 [40] Peleteiro-Alonso J B, Rodríguez-Ojea G, Iglesias-Estévez J. 1995. Spawning control in different turbot (Scophthalmus maximus L.) broodstock groups under artificial and natural photoperiods. In: ICES Marine Science Symposium 201. Copenhagen: ICES, 202–203 [41] Plant T M. 2015. 60 Years of neuroendocrinology: the hypothalamo-pituitary-gonadal axis. Journal of Endocrinology, 226(2): T41–T54 [42] Power D M, Llewellyn L, Faustino M, et al. 2001. Thyroid hormones in growth and development of fish. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 130(4): 447–459 [43] Rad F, Bozaoğlu S, Gözükara S E, et al. 2006. Effects of different long-day photoperiods on somatic growth and gonadal development in Nile tilapia (Oreochromis niloticus L. ). Aquaculture, 255(1–4): 292–300 [44] Reppart S M, Weaver D R, Godson C. 1996. Melatonin receptors step into the light: Cloning and classification of subtypes. Trends in Pharmacological Sciences, 17(3): 100–102 [45] Robert K A, Lesku J A, Partecke J, et al. 2015. Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. Proceedings of the Royal Society B: Biological Sciences, 282(1816): 20151745. doi: 10.1098/rspb.2015.1745 [46] Rocha R M P, Lima L F, Alves A M C V, et al. 2013. Interaction between melatonin and follicle-stimulating hormone promotes in vitro development of caprine preantral follicles. Domestic Animal Endocrinology, 44(1): 1–9 [47] Rodriguez C, Mayo J C, Sainz R M, et al. 2004. Regulation of antioxidant enzymes: a significant role for melatonin. Journal of Pineal Research, 36(1): 1–9 [48] Sébert M E, Legros C, Weltzien F A, et al. 2008. Melatonin activates brain dopaminergic systems in the eel with an inhibitory impact on reproductive function. Journal of Neuroendocrinology, 20(7): 917–929 [49] Sauzet S, Besseau L, Perez P H, et al. 2008. Cloning and retinal expression of melatonin receptors in the European sea bass, Dicentrarchus labrax. General and Comparative Endocrinology, 157(2): 186–195 [50] Shin H S, Kim N N, Lee J, et al. 2011. Diurnal and circadian regulations by three melatonin receptors in the brain and retina of olive flounder Paralichthys olivaceus: profiles following exogenous melatonin. Marine and Freshwater Behaviour and Physiology, 44(4): 223–238 [51] Soares J M, Jr, Masana M I, Erşahin Ç, et al. 2003. Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. The Journal of Pharmacology and Experimental Therapeutics, 306(2): 694–702 [52] Tamura H, Takasaki A, Miwa I, et al. 2008. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. Journal of Pineal Research, 44(3): 280–287 [53] Tan Dunxian, Chen Lidun, Poeggeler B, et al. 1993. Melatonin: A potent endogenous hydroxyl radical scavenger. Journal of Pineal Research, 1: 57–60 [54] Tsutsui K, Ubuka T, Son Y L, et al. 2015. Contribution of GnIH research to the progress of reproductive neuroendocrinology. Frontiers in Endocrinology, 6: 179 [55] Vuilleumier R, Besseau L, Boeuf G, et al. 2006. Starting the zebrafish pineal circadian clock with a single photic transition. Endocrinology, 147(5): 2273–2279 [56] Wright M L. 2002. Melatonin, diel rhythms, and metamorphosis in anuran amphibians. General and Comparative Endocrinology, 126(3): 251–254 [57] Zhang Hongmei, Zhang Yiqiang. 2014. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. Journal of Pineal Research, 57(2): 131–146 [58] Zhao Chunyan, Liu Qinghua, Xu Shihong, et al. 2018a. Identification of type A spermatogonia in turbot (Scophthalmus maximus) using a new cell-surface marker of Lymphocyte antigen 75 (ly75/CD205). Theriogenology, 113: 137–145 [59] Zhao Chunyan, Xu Shihong, Feng Chengcheng, et al. 2018b. Characterization and differential expression of three GnRH forms during reproductive development in cultured turbot Schophthalmus maximus. Journal of Oceanology and Limnology, 36(4): 1360–1373 [60] Zhu Dongmei, Yang Kun, Gul Y, et al. 2014. Effect of photoperiod on growth and gonadal development of juvenile Topmouth Gudgeon Pseudorasbora parva. Environmental Biology of Fishes, 97(2): 147–156 [61] Ziv L, Gothilf Y. 2006. Circadian time-keeping during early stages of development. Proceedings of the National Academy of Sciences of the United States of America, 103(11): 4146–4151 -





 下载:
下载: