-
Abstract: The circadian clock is a fundamental endogenous mechanism of adaptation that coordinates the physiology and behavior of most organisms with diel variations in the external environment to maintain temporal homeostasis. Diatoms are the major primary producers in the ocean. However, little is known about the circadian clock in marine diatoms compared with other organisms. Here, we investigated circadian clock genes, their expression patterns, and responses to environmental stimuli such as light, nitrogen and phosphorus in two marine diatoms, Skeletonema costatum and Phaeodactylum tricornutum, using a combination of qRT-PCR and bioinformatic analysis. We identified 17 and 18 circadian clock genes in P. tricornutum and S. costatum, respectively. Despite significant evolutionary differences, these genes were similar to those of the higher plant Arabidopsis. We also established a molecular model for the marine diatom circadian clock comprising an input pathway, core oscillator, output pathway, and valve effector. Notably, the expression patterns of core clock genes (circadian clock associated 1 (CCA1), late elongated hypocotyl (LHY) and timing of cab 1 (TOC1)) in both species differed from those of terrestrial plants. Furthermore, the expression of these genes was influenced by variations in ambient light, nitrogen and phosphorus availability. Although marine diatoms and higher plants share common circadian clock components, their clock genes have diverged throughout evolution, likely as a result of adapting to contrasting environments.
-
Key words:
- circadian clock /
- circadian genes /
- marine diatoms /
- Phaeodactylum tricornutum /
- Skeletonema costatum
-
Figure 1. Growth curves of Skeletonema costatum and Phaeodactylum tricornutum under control (a and e), continuous light and dark (b and f), N-deplete and N-resupplied (c and g), P-deplete and P-resupplied (d and h) conditions. The red and blue rectangles indicate the sampling time for molecular analysis while the blue rectangles in c, d, g and h indicate the sampling time in the N- or P-resupplied groups. The red arrow indicates the time point of N or P resupply.
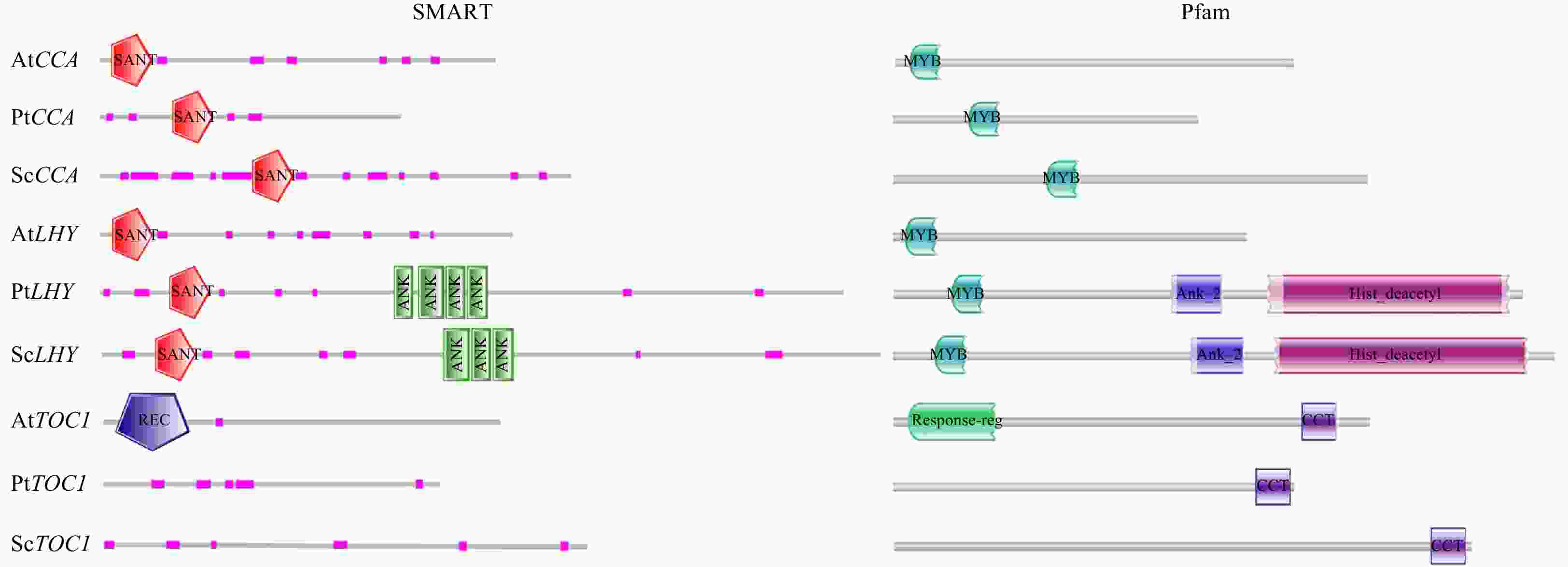
Figure 2. Conservative motif analysis of the core clock genes (CCA1, LHY, and TOC1) in Skeletonema costatum and Phaeodactylum tricornutum based on the databases SMART and Pfam. At is abbreviation of Arabidopsis thaliana; Pt, P. tricornutum; Sc, S. costatum; REC, phosphoacceptor receiver; SANT, switching-defective protein 3 (Swi3), adaptor 2 (Ada2), nuclear receptor co-repressor (N-CoR), transcription factor (TF)IIIB; ANK, ankyrin; Ank2, Ankyrin-B; CCT is timing of cab expression 1.
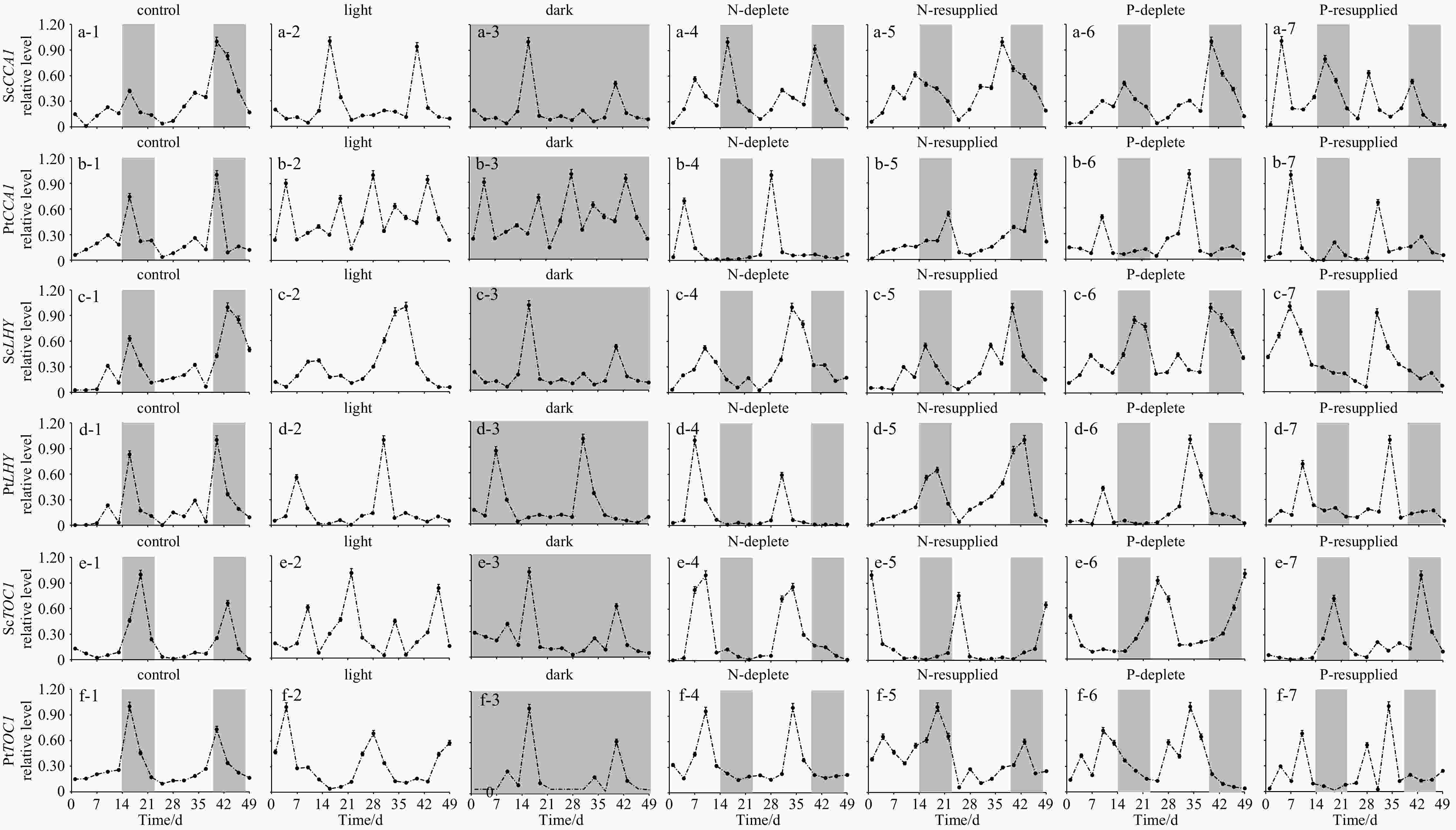
Figure 3. Expression of CCA1, LHY, and TOC1 in Skeletonema costatum and Phaeodactylum tricornutum within 24 h. Expression of CCA1 in S. costatum and P. tricornutum under control (a-1 and b-1), continuous light (a-2 and b-2), continuous dark (a-3 and b-3), N-deplete (a-4 and b-4), N-resupplied (a-5 and b-5), P-deplete (a-6 and b-6) and P-resupplied (a-7 and b-7) conditions; expression of LHY in S. costatum and P. tricornutum under control (c-1 and d-1), continuous light (c-2 and d-2), continuous dark (c-3 and d-3), N-deplete (c-4 and d-4), N-resupplied (c-5 and d-5), P-deplete (c-6 and d-6) and P-resupplied (c-7 and d-7) conditions; and expression of TOC1 in S. costatum and P. tricornutum under control (e-1 and f-1), continuous light (e-2 and f-2), continuous dark (e-3 and f-3), N-deplete (e-4 and f-4), N-resupplied (e-5 and f-5), P-deplete (e-6 and f-6) and P-resupplied (e-7 and f-7) conditions. Sc represents S. costatum; Pt, P. tricornutum.
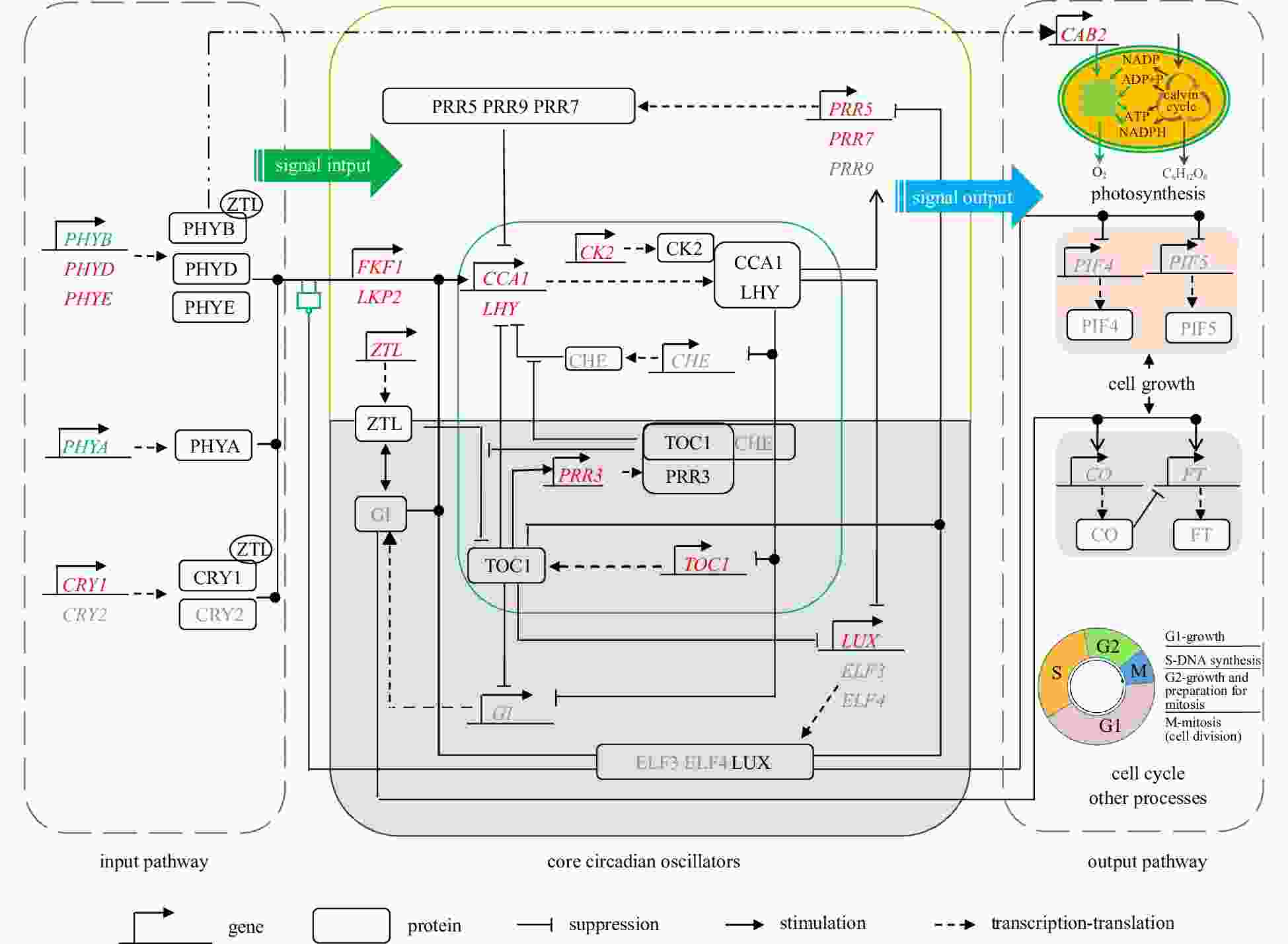
Figure 5. The speculative molecular model of the circadian clock in marine diatoms. Genes in the figure are shown in italics, proteins are shown in normal font. Words in red represent the genes that are identified in Skeletonema costatum and Phaeodactylum tricornutum; words in gray represent the genes and proteins that are not identified in S. costatum and P. tricornutum; words in green represent the genes that are identified in S. costatum; words in blue represent the genes that are identified in P. tricornutum. The core feedback loop of the circadian oscillator is highlighted in green square frame; the white area represents daytime, while the shaded area represents nighttime. The environmental signals of the input pathway and the metabolic process of the output pathways showed in a previous study (Zhang et al., 2016).
Table 1. Circadian clock genes identified in the diatoms Skeletonema costatum, Phaeodactylum tricornutum, and other speciesa
Location Gene name Eukaryotic algae Flowering plantsb Skeletonema costatum Phaeodactylum tricornutum Ostreococcus tauri Chlamydomonas reinhardtii Symbiodinium spp. Cyanidioschyzon merolae Arabidopsis thaliana Oryza sativa Input pathway PHYA + nd nd nd − nd + + PYHB + nd nd nd − nd + + PHYC + + nd nd +c nd + + PHYD + + nd nd − nd + + PHYE + + nd nd − nd + + CRY1 + + nd + + + + + ZTL + + nd nd nd nd + + LKP2 + + nd nd nd nd + + FKF1 + + nd nd nd nd + + Core oscillator CCA1 + + + nd nd nd + + LHY + + nd nd nd nd + nd TOC1 + + + + nd nd + nd PRR3 + + nd nd nd nd + nd PRR5 + + nd nd nd nd + nd PRR7 + + nd nd nd nd + nd PRR9 − + nd nd nd nd + nd CK2 + + + + nd nd + + Output pathway CAB2 + + nd nd nd nd + + Valve effector LUX + + nd nd nd nd + nd Note: aProkaryotes are not included in the table because they have completely different clock genes; bonly representative flowering plants are listed; cunknown type. + indicates detected; nd, not detected; −, no data. The content of this table refer to Martínez-García et al. (2000), Noordally and Millar (2015) and Zhang et al. (2016). -
[1] Alabadí D, Oyama T, Yanovsky M J, et al. 2001. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science, 293(5531): 880–883. doi: 10.1126/science.1061320 [2] Annunziata R, Ritter A, Fortunato A E, et al. 2019. bHLH-PAS protein RITOM1 regulates diel biological rhythms in the marine diatom Phaeodactylum tricornutum. Proceedings of the National Academy of Sciences, 116(26): 13137–13142. doi: 10.1073/pnas.1819660116 [3] Basu D, Dehesh K, Schneider-Poetsch H J, et al. 2000. Rice PHYC gene: structure, expression, map position and evolution. Plant Molecular Biology, 44(1): 27–42. doi: 10.1023/a:1006488119301 [4] Boxall S F, Foster J M, Bohnert H J, et al. 2005. Conservation and divergence of circadian clock operation in a stress-inducible crassulacean acid metabolism species reveals clock compensation against stress. Plant Physiology, 137(3): 969–982. doi: 10.1104/pp.104.054577 [5] Bruce V G. 1970. The biological clock in Chlamydomonas reinhardi. The Journal of Eukaryotic Microbiology, 17(2): 328–334. doi: 10.1111/j.1550-7408.1970.tb02380.x [6] Dehesh K, Tepperman J, Christensen A H, et al. 1991. phy B is evolutionarily conserved and constitutively expressed in rice seedling shoots. Molecular and General Genetics, 225(2): 305–313. doi: 10.1007/BF00269863 [7] Demarsy E, Fankhauser C. 2009. Higher plants use LOV to perceive blue light. Current Opinion in Plant Biology, 12(1): 69–74. doi: 10.1016/j.pbi.2008.09.002 [8] Derveaux S, Vandesompele J, Hellemans J. 2010. How to do successful gene expression analysis using real-time PCR. Methods, 50(4): 227–230. doi: 10.1016/j.ymeth.2009.11.001 [9] Dodd A N, Salathia N, Hall A, et al. 2005. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science, 309(5734): 630–633. doi: 10.1126/science.1115581 [10] Dong M A, Farré E M, Thomashow M F. 2011. Circadian clock-associated 1 and late elongated hypocotyl regulate expression of the c-repeat binding factor (CBF) pathway in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 108(17): 7241–7246. doi: 10.1073/pnas.1103741108 [11] Falkowski P G, Barber R T, Smetacek V. 1998. Biogeochemical controls and feedbacks on ocean primary production. Science, 281(5374): 200–206. doi: 10.1126/science.281.5374.200 [12] Feldman J F, Hoyle M N. 1973. Isolation of circadian clock mutants of Neurospora crassa. Genetics, 75(4): 605–613. doi: 10.1093/genetics/75.4.605 [13] Field C B, Behrenfeld M J, Randerson J T, et al. 1998. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science, 281(5374): 237–240. doi: 10.1126/science.281.5374.237 [14] Filonova A, Haemsch P, Gebauer C, et al. 2013. Protein disulfide isomerase 2 of Chlamydomonas reinhardtii is involved in circadian rhythm regulation. Molecular Plant, 6(5): 1503–1517. doi: 10.1093/mp/sst048 [15] Fujiwara S, Oda A, Yoshida R, et al. 2008. Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering in Arabidopsis. The Plant Cell, 20(11): 2960–2971. doi: 10.1105/tpc.108.061531 [16] Goldman J C. 1993. Potential role of large oceanic diatoms in new primary production. Deep-Sea Research Part I: Oceanographic Research Papers, 40(1): 159–168. doi: 10.1016/0967-0637(93)90059-C [17] Greenham K, McClung C R. 2015. Integrating circadian dynamics with physiological processes in plants. Nature Reviews Genetics, 16(10): 598–610. doi: 10.1038/nrg3976 [18] Harmer S L. 2009. The circadian system in higher plants. Annual Review of Plant Biology, 60(1): 357–377. doi: 10.1146/annurev.arplant.043008.092054 [19] Harmer S L, Hogenesch J B, Straume M, et al. 2000. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science, 290(5499): 2110–2113. doi: 10.1126/science.290.5499.2110 [20] Hazen S P, Schultz T F, Pruneda-Paz J L, et al. 2005. LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proceedings of the National Academy of Sciences of the United States of America, 102(29): 10387–10392. doi: 10.1073/pnas.0503029102 [21] Hotta C T, Gardner M J, Hubbard K E, et al. 2007. Modulation of environmental responses of plants by circadian clocks. Plant, Cell & Environment, 30(3): 333–349, [22] Irigoien X, Huisman J, Harris R P. 2004. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature, 429(6994): 863–867. doi: 10.1038/nature02593 [23] Izawa T, Takahashi Y, Yano M. 2003. Comparative biology comes into bloom: genomic and genetic comparison of flowering pathways in rice and Arabidopsis. Current Opinion in Plant Biology, 6(2): 113–120. doi: 10.1016/S1369-5266(03)00014-1 [24] Kaczorowski K A, Quail P H. 2003. Arabidopsis PSEUDO-RESPONSE REGULATOR7 is a signaling intermediate in phytochrome-regulated seedling deetiolation and phasing of the circadian clock. The Plant Cell, 15(11): 2654–2665. doi: 10.1105/tpc.015065 [25] Kang Lee-Kuo, Tsui Feng-Hsiu, Chang Jeng. 2012. Quantification of diatom gene expression in the sea by selecting uniformly transcribed mRNA as the basis for normalization. Applied and Environmental Microbiology, 78(17): 6051–6058. doi: 10.1128/AEM.00935-12 [26] Kevei É, Nagy F. 2003. Phytochrome controlled signalling cascades in higher plants. Physiologia Plantarum, 117(3): 305–313. doi: 10.1034/j.1399-3054.2003.00049.x [27] Kondo T, Tsinoremas N F, Golden S S, et al. 1994. Circadian clock mutants of cyanobacteria. Science, 266(5188): 1233–1236. doi: 10.1126/science.7973706 [28] Konopka R J, Benzer S. 1971. Clock mutants of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America, 68(9): 2112–2116. doi: 10.1073/pnas.68.9.2112 [29] Lin Chentao, Shalitin D. 2003. Cryptochrome structure and signal transduction. Annual Review of Plant Biology, 54(1): 469–496. doi: 10.1146/annurev.arplant.54.110901.160901 [30] Liu Hua, Wang Honggui, Gao Pengfei, et al. 2009. Analysis of clock gene homologs using unifoliolates as target organs in soybean (Glycine max). Journal of Plant Physiology, 166(3): 278–289. doi: 10.1016/j.jplph.2008.06.003 [31] Lu S X, Liu Hongtao, Knowles S M, et al. 2011. A role for protein kinase casein kinase 2 α-subunits in the Arabidopsis circadian clock. Plant Physiology, 157(3): 1537–1545. doi: 10.1104/pp.111.179846 [32] Más P, Kim W Y, Somers D E, et al. 2003. Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature, 426(6966): 567–570. doi: 10.1038/nature02163 [33] Martínez-García JF, Huq E, Quail PH. 2000. Direct targeting of light signals to a promoter element-bound transcription factor. Science, 288: 859–863. doi: 10.1126/science.288.5467.859 [34] Matsuo T, Okamoto K, Onai K, et al. 2008. A systematic forward genetic analysis identified components of the Chlamydomonas circadian system. Genes & Development, 22(7): 918–930. doi: 10.1101/gad.1650408 [35] Matsushika A, Murakami M, Ito S, et al. 2007. Characterization of circadian-associated pseudo-response regulators: I. Comparative studies on a series of transgenic lines misexpressing five distinctive PRR genes in Arabidopsis thaliana. Bioscience, Biotechnology, and Biochemistry, 71(2): 527–534, [36] McClung C R. 2001. Circadian rhythms in plants. Annual Review of Plant Physiology and Plant Molecular Biology, 52: 139–162. doi: 10.1146/annurev.arplant.52.1.139 [37] McClung C R. 2011. The genetics of plant clocks. Advances in Genetics, 74: 105–139. doi: 10.1016/B978-0-12-387690-4.00004-0 [38] Meyer H, Thienel U, Piechulla B. 1989. Molecular characterization of the diurnal/circadian expression of the chlorophyll a/b-binding proteins in leaves of tomato and other dicotyledonous and monocotyledonous plant species. Planta, 180(1): 5–15. doi: 10.1007/BF02411404 [39] Michael T P, Salomé P A, Yu H J, et al. 2003. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science, 302(5647): 1049–1053. doi: 10.1126/science.1082971 [40] Millar A J, Carré I A, Strayer C A, et al. 1995. Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science, 267(5201): 1161–1163. doi: 10.1126/science.7855595 [41] Moulager M, Monnier A, Jesson B, et al. 2007. Light-dependent regulation of cell division in Ostreococcus: evidence for a major transcriptional input. Plant Physiology, 144(3): 1360–1369. doi: 10.1104/pp.107.096149 [42] Mulekar J J, Huq E. 2014. Expanding roles of protein kinase CK2 in regulating plant growth and development. Journal of Experimental Botany, 65(11): 2883–2893. doi: 10.1093/jxb/ert401 [43] Murakami M, Ashikari M, Miura K, et al. 2003. The evolutionarily conserved OsPRR quintet: rice pseudo-response regulators implicated in circadian rhythm. Plant and Cell Physiology, 44(11): 1229–1236. doi: 10.1093/pcp/pcg135 [44] Murakami M, Tago Y, Yamashino T, et al. 2007. Comparative overviews of clock-associated genes of Arabidopsis thaliana and Oryza sativa. Plant and Cell Physiology, 48(1): 110–121. doi: 10.1093/pcp/pcl043 [45] Noordally Z B, Millar A J. 2015. Clocks in algae. Biochemistry, 54(2): 171–183. doi: 10.1021/bi501089x [46] Para A, Farré E M, Imaizumi T, et al. 2007. PRR3 is a vascular regulator of TOC1 stability in the Arabidopsis circadian clock. The Plant Cell, 19(11): 3462–3473. doi: 10.1105/tpc.107.054775 [47] Ragni M, d’Alcalà M R. 2007. Circadian variability in the photobiology of Phaeodactylum tricornutum: pigment content. Journal of Plankton Research, 29(2): 141–156. doi: 10.1093/plankt/fbm002 [48] Salomé P A, Weigel D, McClung C R. 2010. The role of the Arabidopsis morning loop components CCA1, LHY, PRR7, and PRR9 in temperature compensation. The Plant Cell, 22(11): 3650–3661. doi: 10.1105/tpc.110.079087 [49] Salter M G, Franklin K A, Whitelam G C. 2003. Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature, 426(6967): 680–683. doi: 10.1038/nature02174 [50] Sato E, Nakamichi N, Yamashino T, et al. 2002. Aberrant expression of the Arabidopsis circadian-regulated APRR5 gene belonging to the APRR1/TOC1 quintet results in early flowering and hypersensitiveness to light in early photomorphogenesis. Plant and Cell Physiology, 43(11): 1374–1385. doi: 10.1093/pcp/pcf166 [51] Schaffer R, Ramsay N, Samach A, et al. 1998. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell, 93(7): 1219–1229. doi: 10.1016/S0092-8674(00)81465-8 [52] Schweiger E, Wallraff H G, Schweiger H G. 1964. Endogenous circadian rhythm in cytoplasm of Acetabularia: influence of the nucleus. Science, 146(3644): 658–659. doi: 10.1126/science.146.3644.658 [53] Seo S, Kim J, Lee J W, et al. 2020. Enhanced pyruvate metabolism in plastids by overexpression of putative plastidial pyruvate transporter in Phaeodactylum tricornutum. Biotechnology for Biofuels, 13: 120. doi: 10.21203/rs.3.rs-25111/v1 [54] Staiger D. 2002. Circadian rhythms in Arabidopsis: time for nuclear proteins. Planta, 214(3): 334–344. doi: 10.1007/s004250100662 [55] Strayer C, Oyama T, Schultz T F, et al. 2000. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science, 289(5480): 768–771. doi: 10.1126/science.289.5480.768 [56] Troein C, Locke J C W, Turner M S, et al. 2009. Weather and seasons together demand complex biological clocks. Current Biology, 19(22): 1961–1964. doi: 10.1016/j.cub.2009.09.024 [57] Turek F W, Joshu C, Kohsaka A, et al. 2005. Obesity and metabolic syndrome in circadian Clock mutant mice. Science, 308(5724): 1043–1045. doi: 10.1126/science.1108750 [58] Wada T, Shimono K, Kikukawa T, et al. 2011. Crystal structure of the eukaryotic light-driven proton-pumping rhodopsin, Acetabularia rhodopsin II, from marine alga. Journal of Molecular Biology, 411(5): 986–998. doi: 10.1016/j.jmb.2011.06.028 [59] Wang Zhiyong, Tobin E M. 1998. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell, 93(7): 1207–1217. doi: 10.1016/S0092-8674(00)81464-6 [60] Wang Dazhi, Zhang Yingjiao, Zhang Shufei, et al. 2013. Quantitative proteomic analysis of cell cycle of the dinoflagellate Prorocentrum donghaiense (Dinophyceae). PLoS ONE, 8(5): e63659. doi: 10.1371/journal.pone.0063659 [61] Wright K P, Mchill A W, Birks B R, et al. 2013. Entrainment of the human circadian clock to the natural light-dark cycle. Current Biology, 23(16): 1554–1558. doi: 10.1016/j.cub.2013.06.039 [62] Zhang Shufeng, Yuan Chunjuan, Chen Ying, et al. 2016. Comparative transcriptomic analysis reveals novel insights into the adaptive response of Skeletonema costatum to changing ambient phosphorus. Frontiers in Microbiology, 7: 1476. doi: 10.3389/fmicb.2016.01476 -





 下载:
下载: