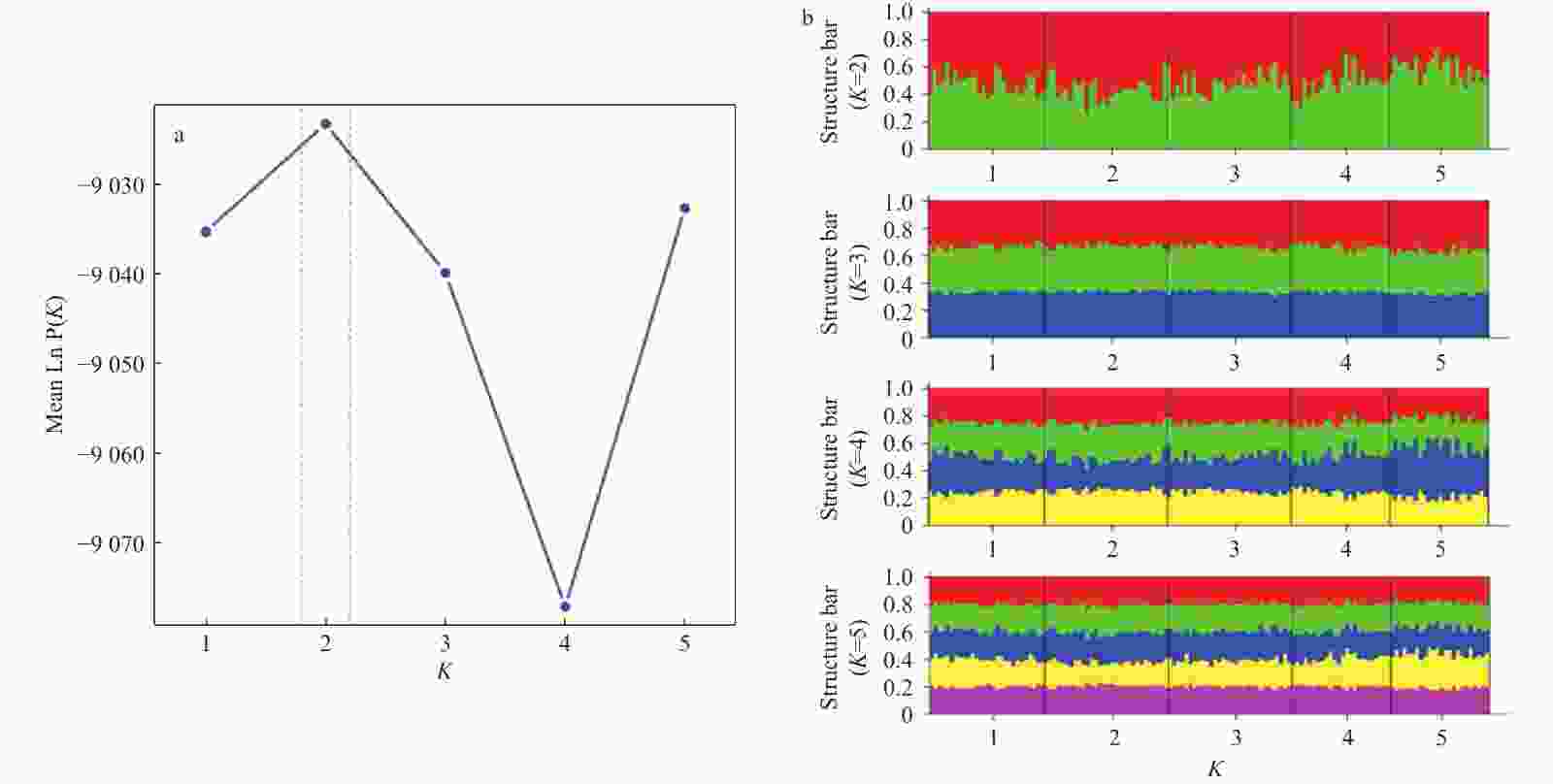
Discordant patterns of genetic variation between mitochondrial and microsatellite markers in Acanthogobius ommaturus across the coastal areas of China
-
Abstract: Acanthogobius ommaturus, which belongs to the family Gobiidae, is a euryhaline and demersal fish that is widely distributed in the coastal areas, harbors, and estuaries of China, D. P. R. Korea and Japan. In this study, the genetic diversity and genetic structure of five geographical populations of A. ommaturus was assessed using the mitochondrial hypervariable region gene and microsatellite markers. The results of the two genetic markers indicated that the A. ommaturus populations had a high level of genetic diversity. The mitochondrial marker detected weak genetic differentiation among populations, and the Neighbor-Joining tree showed that there was no obvious pedigree branches and geographic structure as well. However, population of Zhoushan showed significant genetic differentiation with other populations by microsatellite markers. The population of A. ommaturus has not experienced bottleneck effect recently. We speculated that the Pleistocene climate change and juvenile fish dispersal played an important role in the population differentiation of A. ommaturus.
-
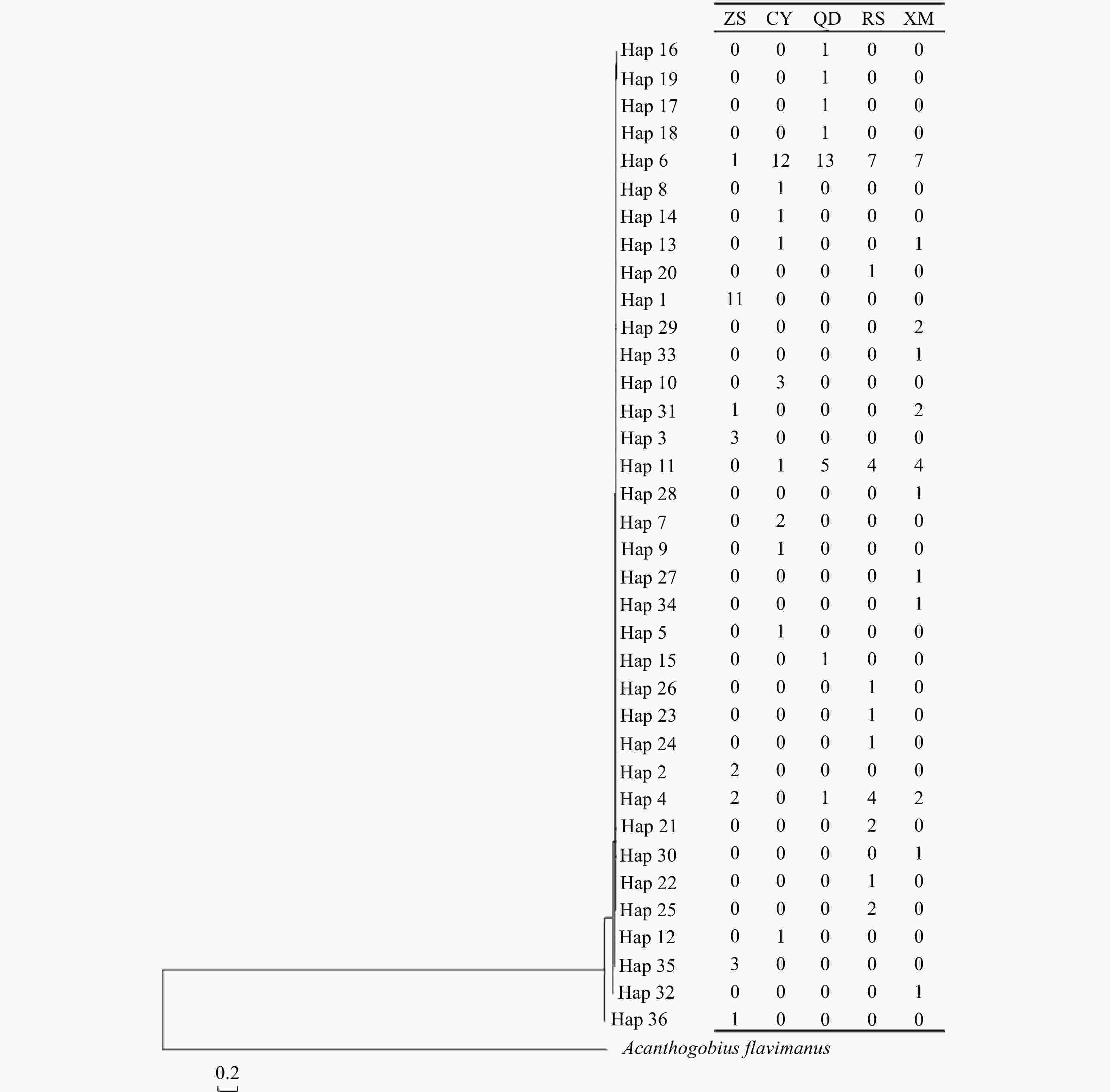
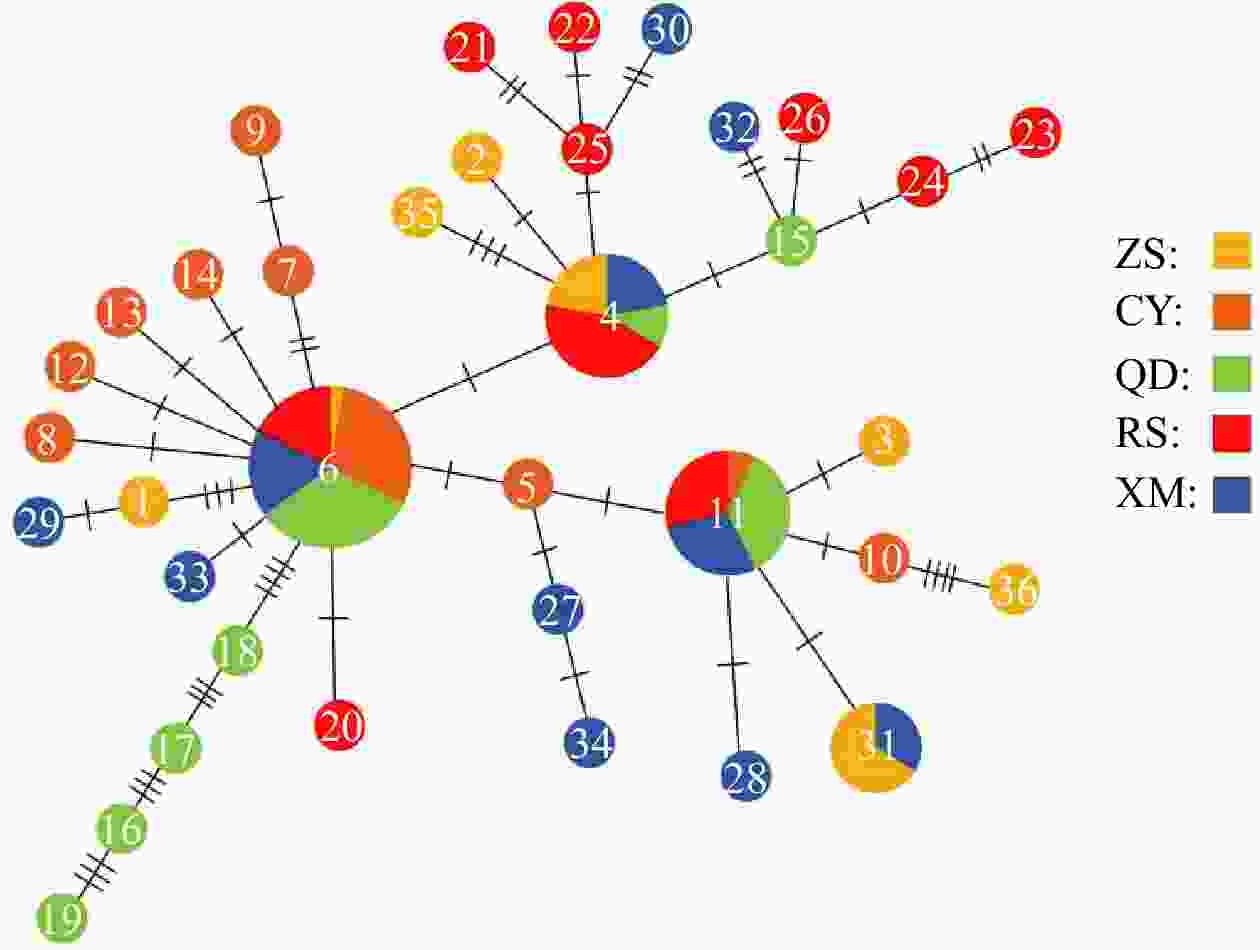
Figure 2. Unrooted Neighbor-joining tree constructed using Kimura-2-parameter for 36 haplotypes of Acanthogobius ommaturus based on mitochondrial DNA hypervariable region analysis. On the right was the number of haplotypes in each population. ZS: Zhoushan; CY: Changyi; QD: Qingdao; RS: Rushan; XM: Xiamen.
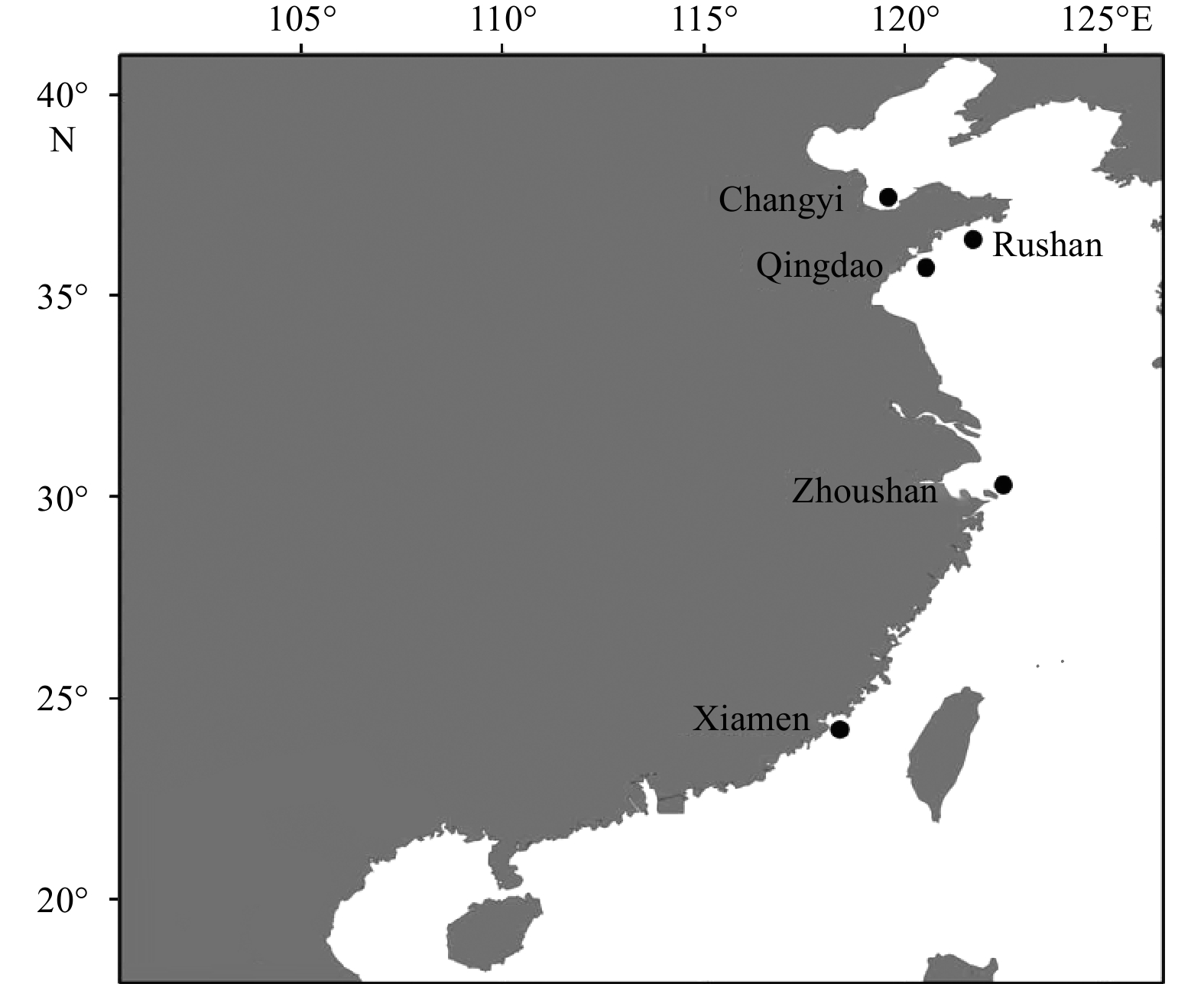
Table 1. Sample information, genetic statistics for the mitochondrial DNA (mtDNA) and smation for simple sequence repeat (SSR) of Acanthogobius ommaturus
Sample locality Abbreviation Sample time Sample number for mtDNA Haplotypenumber Number of polymorphic sites Haplotype diversity (h) Nucleotide diversity (π) Sample number for SSR Zhoushan ZS 2018.01 24 8 16 0.772±0.078 0.008±0.005 30 Changyi CY 2007.09 24 10 10 0.746±0.091 0.003±0.002 28 Qingdao QD 2018.10 24 8 10 0.681±0.091 0.005±0.003 24 Rushan RS 2017.10 24 10 10 0.873±0.044 0.005±0.003 24 Xiamen XM 2017.10 24 12 16 0.891±0.046 0.006±0.004 30 Total / / 120 36 38 0.793±0.070 0.006±0.004 136 Table 2. Pairwise Fst between Acanthogobius ommaturus populations
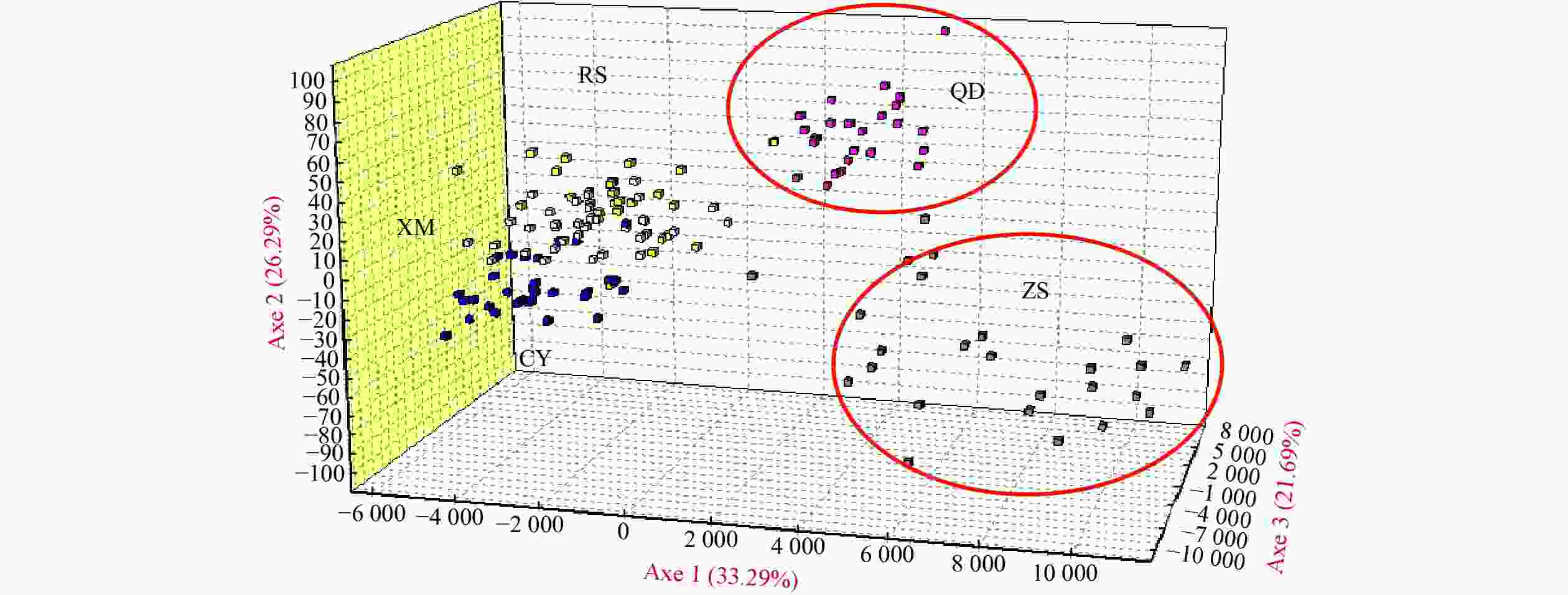
Population ZS CY QD RS CY 0.175* QD 0.184* 0.042 RS 0.160* 0.130* 0.087* XM 0.114* 0.031 0.037 0.052 Note: * significant at P<0.05. ZS: Zhoushan; CY: Changyi; QD: Qingdao; RS: Rushan; XM: Xiamen. Table 3. Pairwise Fst (above diagonal) and (δμ)2 (below diagonal) among populations of Acanthogobius ommaturus
Population RS CY XM ZS QD RS / 0.0185* 0.0083 0.0235* 0.0222* CY 0.781 / 0.0061 0.0306* 0.0440 XM 1.320 0.951 / 0.0240* 0.0283* ZS 3.883 5.171 6.277 / 0.0229* QD 2.815 3.646 2.415 8.726 / Note: * significant at P<0.05. ZS: Zhoushan; CY: Changyi; QD: Qingdao; RS: Rushan; XM: Xiamen. Table 4. Results of Wilcoxon’s heterozygosity excess test, mode shift indicator for a genetic bottleneck in five populations of Acanthogobius ommaturus
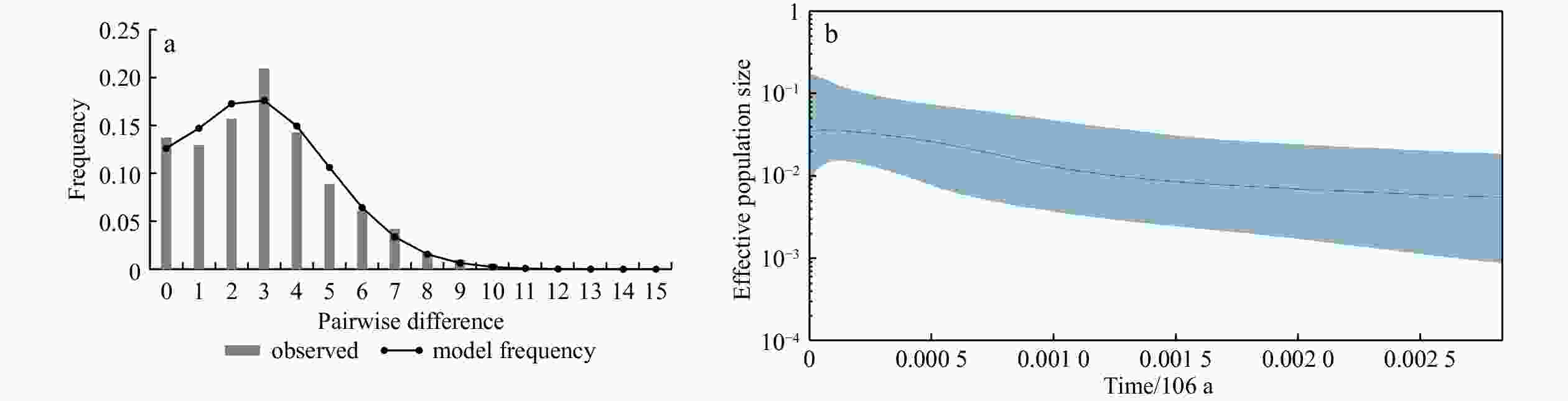
Population Wilcoxon sign-rank test Mode shift IAM TPM SMM RS 0.99170 1.00000 1.00000 L CY 0.50000 0.99991 0.99994 L XM 0.61957 0.99994 0.99994 L ZS 0.50000 0.99979 0.99994 L QD 0.98236 1.00000 1.00000 L Note: RS: Rushan; CY: Changyi; XM: Xiamen; QD: Qingdao; ZS: Zhoushan. -
Avise J C. 2000. Phylogeography: The History and Formation of Species. 3rd ed. Cambridge, MA: Harvard University Press, 301–447 Belkhir K, Borsa P, Chikhi L, et al. 2004. GENETIX 4.05, logiciel sous Windows TM pour la génétique des populations. Laboratoire Génome, Populations, Interactions, CNRS UMR 5000. Montpellier: Université de Montpellier II Bohonak A J. 2002. IBD (isolation by distance): a program for analyses of isolation by distance. Journal of Heredity, 93(2): 153–154. doi: 10.1093/jhered/93.2.153 Cann R L, Brown W M, Wilson A C. 1984. Polymorphic sites and the mechanism of evolution in human mitochondrial DNA. Genetics, 106(3): 479–499. doi: 10.1093/genetics/106.3.479 Cao Yan. 2016. Genetic diversity of 3 sparid species in coastal waters of China based on mitochondrial control region sequences (in Chinese)[dissertation]. Guangzhou: Jinan University Cavalli-Sforza L L. 1998. The DNA revolution in population genetics. Trends in Genetics, 14(2): 60–65. doi: 10.1016/S0168-9525(97)01327-9 Cunningham K M, Canino M F, Spies I B, et al. 2009. Genetic isolation by distance and localized fjord population structure in Pacific cod (Gadus macrocephalus): limited effective dispersal in the northeastern Pacific Ocean. Canadian Journal of Fisheries and Aquatic Sciences, 66(1): 153–166. doi: 10.1139/F08-199 DiBattista J D, Travers M J, Moore G I, et al. 2017. Seascape genomics reveals fine-scale patterns of dispersal for a reef fish along the ecologically divergent coast of northwestern Australia. Molecular Ecology, 26(22): 6206–6223. doi: 10.1111/mec.14352 Dong Zhaoke, Wang Yangzhou, Li Chao, et al. 2021. Mitochondrial DNA as a molecular marker in insect ecology: current status and future prospects. Annals of the Entomological Society of America, 114(4): 470–476. doi: 10.1093/aesa/saab020 Drummond A J, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology, 7: 214. doi: 10.1186/1471-2148-7-214 Ellegren H. 2004. Microsatellites: simple sequences with complex evolution. Nature Reviews Genetics, 5(6): 435–445. doi: 10.1038/nrg1348 Evanno G, Regnaut S, Goudet J. 2005. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology, 14(8): 2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x Excoffier L, Lischer H E L. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources, 10(3): 564–567. doi: 10.1111/j.1755-0998.2010.02847.x Fan Haiyang, Ji Yupeng, Zhang Shihua, et al. 2005. Research of fishery biology of the neritic fish Synechogobius ommaturus in the area of the Huanghe delta. Periodical of Ocean University of China (in Chinese), 35(5): 733–736 Fan Xiaoteng, Wang Luanjin, Wei Xuefeng, et al. 2021. The impairment of continuous malnutrition on larval fish swimming performance at the mouth-opening stage. Aquaculture, 544: 15. doi: 10.1016/j.aquaculture.2021.737053 Feng Jian, Zhu Junquan, Zheng Zhongming, et al. 2004. Studies on the individual fecundity of Synechogobius hasta. Journal of Zhejiang Ocean University: Natural Science (in Chinese), 23(4): 302–305, 314 Ferreira D G, Galindo B A, Frantine-Silva W, et al. 2015. Genetic structure of a Neotropical sedentary fish revealed by AFLP, microsatellite and mtDNA markers: a case study. Conservation Genetics, 16(1): 151–166. doi: 10.1007/s10592-014-0648-2 Frankham R, Ballou J D, Briscoe D A. 2002. Introduction to Conservation Genetics. Cambridge: Cambridge University Press Gao Tianxiang, Gao Bingbing, Li Zhonglu, et al. 2020. Population genetics study of Leiognathus equulus based on the control region fragment of mitochondrial DNA. Journal of Fisheries of China (in Chinese), 44(5): 715–722 Gao Tianxiang, Li Lin, Fang Rundong, et al. 2019. Shallow genetic structure of Pholis fangi in Bohai Sea and Yellow Sea inferred from mtDNA control region. Journal of Ocean University of China, 18(4): 947–952. doi: 10.1007/s11802-019-3991-6 Gharesouran J, Hosseinzadeh H, Ghafouri-Fard S, et al. 2021. STRs: ancient architectures of the genome beyond the sequence. Journal of Molecular Neuroscience, 71(12): 2441–2455. doi: 10.1007/s12031-021-01850-6 Goudet J. 2005. FSTAT. http://www2.unil.ch/popgen/softwares/fstat.htm[2005-08-23] Grant W A S, Bowen B W. 1998. Shallow population histories in deep evolutionary lineages of marine fishes: insights from sardines and anchovies and lessons for conservation. Journal of Heredity, 89(5): 415–426. doi: 10.1093/jhered/89.5.415 Guo Jianzhong. 2018. The community structure and particle size spectrum of fish in Daya Bay (in Chinese)[dissertation]. Shanghai: Shanghai Ocean University Guo Xinhong, Liu Shaojun, Liu Qiao, et al. 2004. New progresses on mitochondrial DNA in fish. Acta Genetica Sinica (in Chinese), 31(9): 983–1000 He Lijun, Zhang Aibing, Weese D, et al. 2014. Demographic response of cutlassfish (Trichiurus japonicus and T. nanhaiensis) to fluctuating palaeo-climate and regional oceanographic conditions in the China Seas. Scientific Reports, 4: 6380. doi: 10.1038/srep06380 Hewitt G. 2000. The genetic legacy of the Quaternary ice ages. Nature, 405(6789): 907–913. doi: 10.1038/35016000 Hwang Jiang-Shiou, Wong C-Kim. 2005. The China coastal current as a driving force for transporting Calanus sinicus (Copepoda: Calanoida) from its population centers to waters off Taiwan and Hong Kong during the winter northeast monsoon period. Journal of Plankton Research, 27(2): 205–210. doi: 10.1093/plankt/fbh162 Jaafar T N A M, Taylor M I, Nor S A M, et al. 2020. Comparative genetic stock structure in three species of commercially exploited Indo-Malay Carangidae (Teleosteii, Perciformes). Journal of Fish Biology, 96(2): 337–349. doi: 10.1111/jfb.14202 Jensen J L, Bohonak A J, Kelley S T. 2005. Isolation by distance, web service. BMC Genetics, 6: 13 Jin Xiaoxiao. 2013. Complete mitochondrial genome and phylogenetic analysis of 16 gobies in East China Sea (in Chinese)[dissertation]. Zhoushan: Zhejiang Ocean University Kanakachari M, Chatterjee R N, Rajkumar U, et al. 2020. Indian Red Jungle fowl depicts close genetic relationship with Indian native chicken breeds as evidenced through whole mitochondrial genome intersection. Conservation Genetics, Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7): 1870–1874. doi: 10.1093/molbev/msw054 Lane H S, Symonds J E, Ritchie P A. 2016. The phylogeography and population genetics of Polyprion oxygeneios based on mitochondrial DNA sequences and microsatellite DNA markers. Fisheries Research, 174: 19–29. doi: 10.1016/j.fishres.2015.08.009 Li Yuan, Chen Xiao, Wu Kun, et al. 2020. Characterization of simple sequence repeats (SSRs) in ciliated protists inferred by comparative genomics. Microorganisms, 8(5): 662. doi: 10.3390/microorganisms8050662 Li Yuan, Lin Longshan, Song Na, et al. 2018. Population genetics of Pampus echinogaster along the Pacific coastline of China: insights from the mitochondrial DNA control region and microsatellite molecular markers. Marine and Freshwater Research, 69(6): 971–981. doi: 10.1071/MF17218 Liu Jinxian, Gao Tianxiang, Yokogawa K, et al. 2006. Differential population structuring and demographic history of two closely related fish species, Japanese sea bass (Lateolabrax japonicus) and spotted sea bass (Lateolabrax maculatus) in northwestern Pacific. Molecular Phylogenetics and Evolution, 39(3): 799–811. doi: 10.1016/j.ympev.2006.01.009 Lu Lifeng, Zhang Qun, Yang Xishu, et al. 2018. Genetic variation of Chelidonichthys kumu in the coastal waters of China based on mtDNA control region sequences. Marine Fisheries (in Chinese), 40(3): 257–264 Luo Zhi, Li Xiaodong, Bai Haijuan, et al. 2008. Comparison on nutrient composition and morphology between wild and cultured Synechogobius hasta. Journal of Shanghai Fisheries University (in Chinese), 17(2): 182–186 Meng Wei. 2019. Study on the phylogeographic pattern of two specialized grades schizothoracine fishes (in Chinese)[dissertation]. Urumqi: Xinjiang University Morgan T D, Graham C F, McArthur A G, et al. 2018. Genetic population structure of the round whitefish (Prosopium cylindraceum) in North America: multiple markers reveal glacial refugia and regional subdivision. Canadian Journal of Fisheries and Aquatic Sciences, 75(6): 836–849. doi: 10.1139/cjfas-2016-0528 Nei M. 1975. Molecular population genetics and evolution. Frontiers in Microbiology, 40: 1–288. doi: 10.1016/0014-5793(77)80772-2 Nei M. 1987. Molecular Evolutionary Genetics. New York: Columbia University Ovenden J R, Salini J, O’connor S, et al. 2004. Pronounced genetic population structure in a potentially vagile fish species (Pristipomoides multidens, Teleostei; Perciformes; Lutjanidae) from the East Indies triangle. Molecular Ecology, 13(7): 1991–1999. doi: 10.1111/j.1365-294X.2004.02210.x Palumbi S R. 1994. Genetic divergence, reproductive isolation, and marine speciation. Annual Review of Ecology and Systematics, 25: 547–572. doi: 10.1146/annurev.es.25.110194.002555 Palumbi S R. 2003. Population genetics, demographic connectivity, and the design of marine reserves. Ecological Applications, 13(sp1): 146–158. doi: 10.1890/1051-0761(2003)013[0146:PGDCAT]2.0.CO;2 Piry S, Luikart G, Cornuet J M. 1999. Computer note. BOTTLENECK: a computer program for detecting recent reductions in the effective population size using allele frequency data. Journal of Heredity, 90(4): 502–503. doi: 10.1093/jhered/90.4.502 Pritchard J K, Stephens M, Onnelly P D. 2000. Inference of population structure using multilocus genotype data. Genetics, 155(2): 945–959. doi: 10.1093/genetics/155.2.945 Raymond M, Rousset F. 1995. GENEPOP (Version 1.2): population genetics software for exact tests and ecumenicism. Journal of Heredity, 86(3): 248–249. doi: 10.1093/oxfordjournals.jhered.a111573 Rocha L A, Robertson D R, Roman J, et al. 2005. Ecological speciation in tropical reef fishes. Proceedings of the Royal Society B: Biological Sciences, 272(1563): 573–579. doi: 10.1098/2004.3005 Sambrook J, Fritsch E F, Maniatis T. 1989. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Sharma S P, Ghazi M G, Katdare S, et al. 2021. Microsatellite analysis reveals low genetic diversity in managed populations of the critically endangered gharial (Gavialis gangeticus) in India. Scientific Reports, 11(1): 5627. doi: 10.1038/s41598-021-85201-w Song Chenyu, Feng Ziyi, Li Chunhou, et al. 2020. Profile and development of microsatellite primers for Acanthogobius ommaturus based on high-throughput sequencing technology. Journal of Oceanology and Limnology, 38(6): 1880–1890. doi: 10.1007/s00343-019-9154-1 Song Na, Song Lin, Gao Tianxiang, et al. 2011. Comparative analysis of genetic diversity of Synechogobius ommaturus based on the mitochondrial DNA control region. Journal of Fisheries of China (in Chinese), 35(3): 321–326 Song Na, Zhang Xiumei, Gao Tianxiang. 2010a. Genetic diversity and population structure of spottedtail goby (Synechogobius ommaturus) based on AFLP analysis. Biochemical Systematics and Ecology, 38(6): 1089–1095. doi: 10.1016/j.bse.2010.10.007 Song Na, Zhang Xiumei, Sun Xifu, et al. 2010b. Population genetic structure and larval dispersal potential of spottedtail goby Synechogobius ommaturus in the North-West Pacific. Journal of Fish Biology, 77(2): 388–402. doi: 10.1111/j.1095-8649.2010.02694.x Susanti R, Yuniastuti A, Kartikasari A D. 2021. Genetic characterization of the central Javanese duck using microsatellite markers. Sains Malaysiana, 50(1): 53–61. doi: 10.17576/jsm-2021-5001-06 Waelbroeck C, Labeyrie L, Michel E, et al. 2002. Sea-level and deep water temperature changes derived from benthic foraminifera isotopic records. Quaternary Science Reviews, 21(1–3): 295–305, Wang Xiaodong, Chen Dunxue, Zhou Xianjun, et al. 2016a. Complete mitochondrial genome of javeline goby (Synechogobius hasta). Mitochondrial DNA Part A, 27(6): 4586–4587. doi: 10.3109/19401736.2014.987254 Wang Miao, Hong Bo, Sun Zhenzhong. 2016b. Fish stock density and diversity: spatial and temporal distribution in the outlet of Hangzhou Bay in spring and summer. Chinese Agricultural Science Bulletin (in Chinese), 32(20): 11–16 Wang Jinxing, Zhao Xiaofan. 1994. Chromosome studies of Synechogobius ommaturus (Osteichthyes: Gobiidae). Marine Science (in Chinese), (4): 47–50 Wright J M, Bentzen P. 1994. Microsatellites: genetic markers for the future. Reviews in Fish Biology and Fisheries, 4(3): 384–388. doi: 10.1007/BF00042912 Wu Renxie, Zhang Haoran, Niu Sufang, et al. 2019. Study on population genetic variation of Trichiurus japonicus in nearshore of the East China Sea in mitochondrial control region sequences. Oceanologia et Limnologia Sinica (in Chinese), 50(6): 1318–1327 Wu Hanlin, Zhong Junsheng. 2008. Osteichthys, Perciformes, Gobioidei. Fauna Sinica, Osteichthys, Perciformes (in Chinese). Beijing: Science Press, 5136–718 Xiao Yongshuang, Ma Daoyuan, Dai Ming, et al. 2016. The impact of Yangtze River discharge on the genetic structure of a population of the rock bream, Oplegnathus fasciatus. Marine Biology Research, 12(4): 426–434. doi: 10.1080/17451000.2016.1154576 Xu Shengyong. 2018. Population genomics and adaptive evolution of Sebastiscus marmoratus (in Chinese)[dissertation]. Qingdao: Ocean University of China Xu Shengyong, Song Na, Zhao Linlin, et al. 2017. Genomic evidence for local adaptation in the ovoviviparous marine fish Sebastiscus marmoratus with a background of population homogeneity. Scientific Reports, 7(1): 1562. doi: 10.1038/s41598-017-01742-z Yang Kun. 2018. Studies on the comparative molecular phylogeography of two frogs in Zhoushan Archipelago (in Chinese)[dissertation]. Hangzhou: China Jiliang University Yang Xishu, Zhang Qun, Xue Dan, et al. 2018. Genetic analysis of Terapon jarbua in coastal waters of China by using mitochondrial DNA control region sequencing. Haiyang Xuebao (in Chinese), 38(5): 1852–1859 Zhang Hanye, Hu Fen. 2005. Spatial heterogeneity of Todarodes pacificus in East China Sea in winter. Chinese Journal of Ecology (in Chinese), 24(11): 1299–1302 Zhang Jing, Qiu Junwen, Chen Chunliang, et al. 2020. Analysis of the fish community structure and its relationship with environmental factors in autumn and spring in northern Dapeng Bay, Guangdong. Journal of Guangdong Ocean University (in Chinese), 40(6): 43–52 Zhang Gongjun, Yang Changping, Liu Yan, et al. 2022. Pattern of fish community and its relationship with environmental factors in Fangchenggang (Qinzhou coastal area of Beibu Gulf). South China Fisheries Science (in Chinese), 18(4): 20–33 Zheng Wei. 2015. Molecular phylogeography study of Charybdis japonica and Charybdis bimaculata in East China Sea and Yellow Sea (in Chinese)[dissertation]. Zhoushan: Zhejiang Ocean University Zhu Meigui, Yang Gang, Zhang Tao, et al. 2016. Feeding habits of Acanthogobius ommaturus in the Yangtze Estuary. Journal of Fishery Sciences of China (in Chinese), 23(4): 914–923 -




 下载:
下载: