-
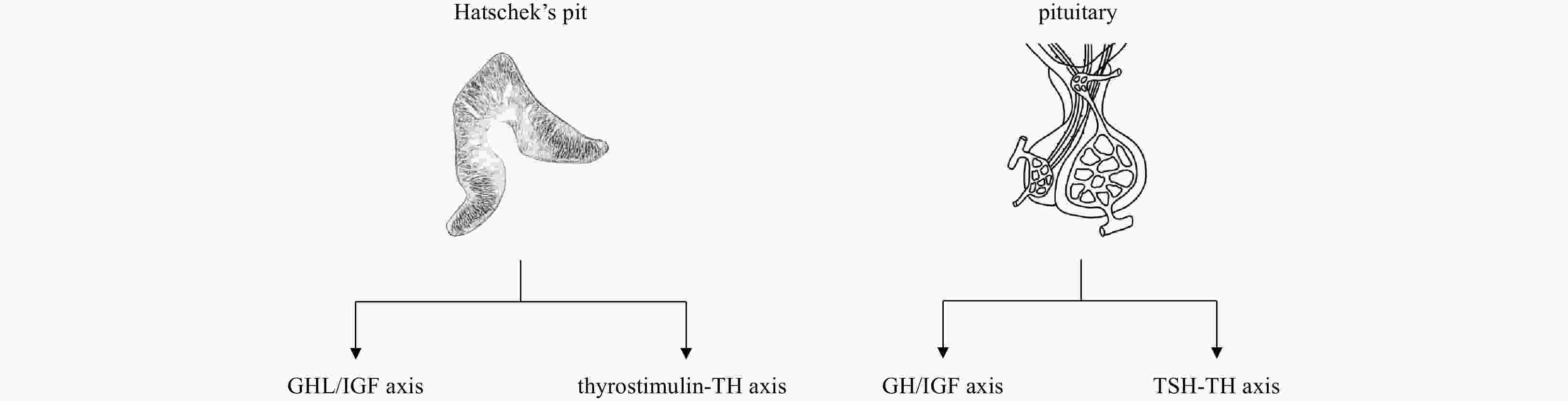
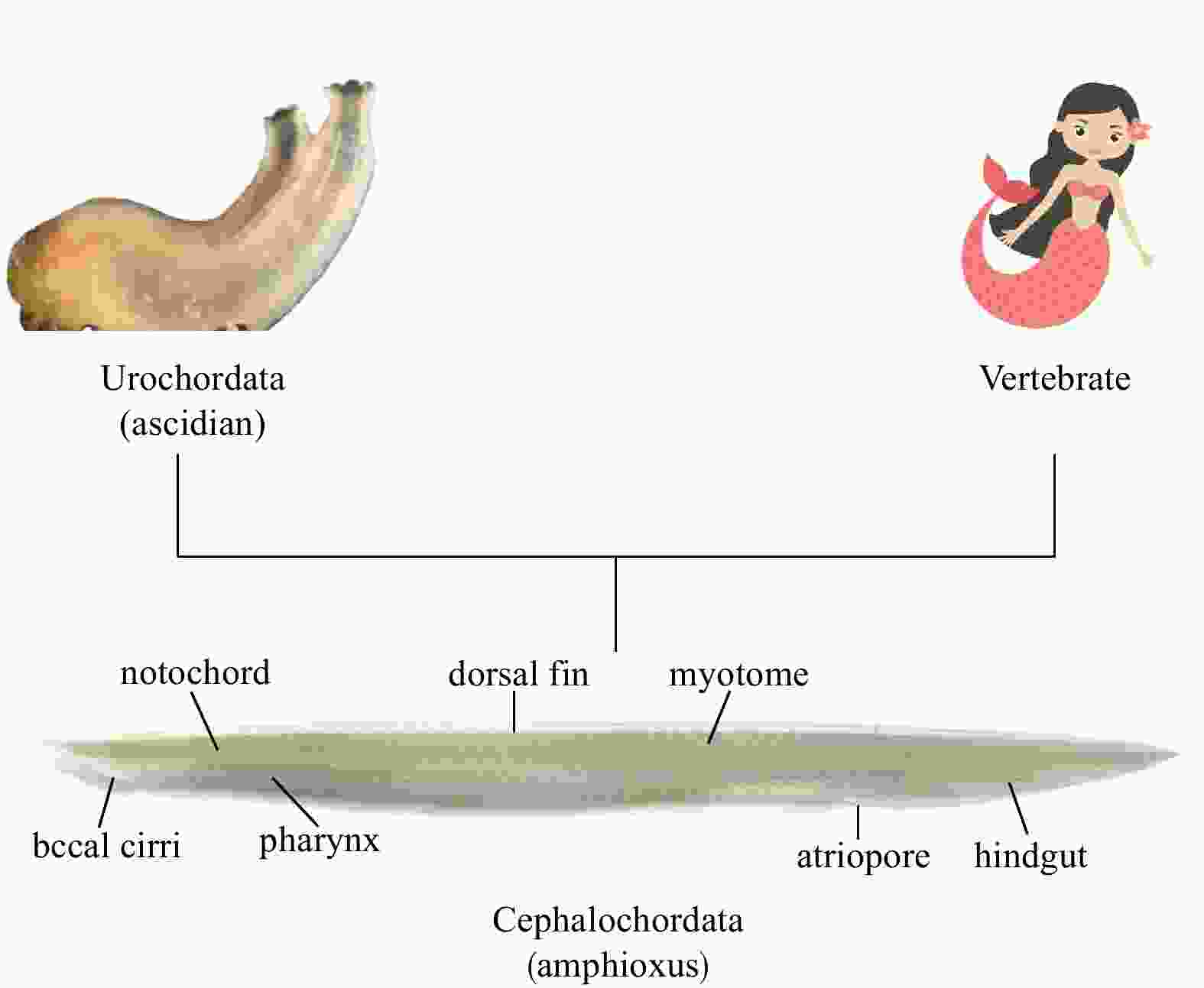
Abstract: Pituitary gland, or pituitary for short, is characteristic of all vertebrates. As a “master gland” controlling a multitude of important functions in the body, its evolutionary origin has been an object of investigations of evolutionary biology for two centuries. Previous morphological, ultrastructural and immunohistochemical studies suggested the homology of the Hatschek’s pit of amphioxus and vertebrate pituitary. Developmental genetics study showed that the development of Hatschek’s pit and vertebrate pituitary is both subject to regulation by the common genes such as Pit1, Lhx3 and BMP3b. Our recent studies demonstrated that the Hatschek’s pit is able to secrete growth hormone (GH)-like hormone and thyroid-stimulating hormone (TSH)-like hormone that both play functions similar to vertebrate GH and TSH. We thus think that the emergence of Hatschek’s pit represents one of important events during endocrine network evolution, which laid a foundation for the subsequent formation of a hypothalamic-pituitary system in vertebrates.
-
Key words:
- protochordate /
- amphioxus /
- brain /
- pituitary /
- evolution
-
Figure 2. Development of vertebrate pituitary (a) and main signalling pathways and factors controlling pituitary development (b). a. Pituitary development starts with the formation of the hypophyseal placode, apposed to the future ventral diencephalon. The placode later invaginates to form Rathke’s pouch (RP), still in contact in its dorsal most part with the infundibulum, which lies within the ventral diencephalon. The infundibulum then evaginates towards RP. The anterior and intermediate lobes originate from RP. b. During pituitary development, the temporal and spatial expression of a cascade of signaling molecules and transcription factors plays a crucial role in organ commitment, cell proliferation, patterning, and terminal differentiation.
-
Alatzoglou K S, Gregory L C, Dattani M T. 2020. Development of the pituitary gland. Comprehensive Physiology, 10(2): 389–413 Barrington E J W. 1963. Hormones and evolution. In: Barrington E J W, ed. An Introduction to General and Comparative Endocrinology. Oxford: Clarendon Press Bateson W. 1885. The later stages in the development of Balanoglossus kowalevskii, with a suggestion as to the affinities of the Enteropneusta. Quarterly Journal of Microscopical Science, 25(S1): 81–122 Candiani S, Holland N D, Oliveri D, et al. 2008. Expression of the amphioxus Pit-1 gene (AmphiPOU1F1/Pit-1) exclusively in the developing preoral organ, a putative homolog of the vertebrate adenohypophysis. Brain Research Bulletin, 75(2–4): 324–330 Candiani S, Pestarino M. 1998. Expression of the tissue-specific transcription factor Pit-1 in the lancelet, Bbranchiostoma lanceolatum. Journal of Comparative Neurology, 392(3): 343–351. doi: 10.1002/(SICI)1096-9861(19980316)392:3<343::AID-CNE5>3.0.CO;2-1 Cattie J T. 1882. Recherches sur la glande pinéale (epihphysis cerebri) des plagiostomes, des ganoïdes et des téléostéens. Arch Biology (in German), 3: 101–194 Chang C Y, Liu Y X, Zhu Y T, et al. 1985. The reproductive endocrinology of amphioxus. In: Carlick D G, Korner P I, eds. Frontiers in Physiological Research. Canberra: Australian Academy of Science, 79–86 Cohen E L, Wondisford E F, Radovick S. 1996. Role of Pit-1 in the gene expression of growth hormone, prolactin, and thyrotropin. Endocrinology and Metabolism Clinics of North America, 25(3): 523–540. doi: 10.1016/S0889-8529(05)70339-X Conklin E G. 1932. The embryology of amphioxus. Journal of Morphology, 54(1): 69–151. doi: 10.1002/jmor.1050540103 Cunningham J T. 1887. Memoirs: Dr. Dohrn’s inquiries into the evolution of organs in the Chordata. Journal of Cell Science, s2-27: 265–284 Davis S W, Mortensen A H, Camper S A. 2011. Birthdating studies reshape models for pituitary gland cell specification. Developmental Biology, 352(2): 215–227. doi: 10.1016/j.ydbio.2011.01.010 Dawid I B, Toyama R, Taira M. 1995. LIM domain proteins. Comptes rendus de l'Académie des sciences. Série III, 318(3): 295–306 Dos Santos S, Bardet C, Bertrand S, et al. 2009. Distinct expression patterns of glycoprotein hormone-α2 and -β5 in a basal chordate suggest independent developmental functions. Endocrinology, 150(8): 3815–3822. doi: 10.1210/en.2008-1743 Drach P. 1948. Embranchcment des Céphaloeordés (in French). In: Traité de Zoologic, ed. Paris: P. P. Grassé Fang Yongqiang. 1993. Immunocytochemical localization of fish gonadotropin (GTH) in the brain vesicle and Hatschek’s pit of amphioxus. Chinese Science Bulletin, 38(20): 1747–1751 Fang Yongqiang, Huang Weiquan, Chen Lei, et al. 1999. Distribution of gonadotropin-releasing hormone in the brain and Hatschek’s pit of amphioxus (Branchiostoma belcheri). Acta Zoologica Sinica, 45(1): 106–111 Glardon S, Holland L Z, Gehring W J, et al. 1998. Isolation and developmental expression of the amphioxus pax-6 gene (amphipax-6): insights into eye and photoreceptor evolution. Development, 125(14): 2701–2710. doi: 10.1242/dev.125.14.2701 Gorbman A, Nozaki M, Kubokawa K. 1999. A brain-Hatschek’s pit connection in amphioxus. General and Comparative Endocrinology, 113(2): 251–254. doi: 10.1006/gcen.1998.7193 Haller B. 1897. Untersuchungen über die hypophyse und die infundibularorgane. Morphologisches Jahrbuch (in German), 25: 31–114 Hatschek B. 1881. Studien über Entwicklung des Amphioxus (in German). Ann Arbor: University of Michigan Library Press, 1–88 Hatschek B. 1884. Mittheilungen über amphioxus. Zoologischer Anzeiger (in German), 7(177): 517–520 Hatschek B. 1892. Die metamerie des amphioxus und des ammocoetes. Anatomischer Anzeiger (in German), 6: 136–161 Hino J, Nishimatsu S I, Nagai T, et al. 2003. Coordination of BMP-3b and cerberus is required for head formation of Xenopus embryos. Developmental Biology, 260(1): 138–157. doi: 10.1016/S0012-1606(03)00223-9 Holland L Z, Albalat R, Azumi K, et al. 2008. The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Research, 18(7): 1100–1111. doi: 10.1101/gr.073676.107 Holland L Z, Holland N D. 2001. Evolution of neural crest and placodes: amphioxus as a model for the ancestral vertebrate?. The Journal of Anatomy, 199(1–2): 85–98 Holland L Z, Schubert M, Kozmik Z, et al. 1999. AmphiPax3/7, an amphioxus paired box gene: insights into chordate myogenesis, neurogenesis, and the possible evolutionary precursor of definitive vertebrate neural crest. Evolution & Development, 1(3): 153–165 Holmberg K. 1982. The ciliated brain duct of Oikopleura dioica (Tunicata, Appendicularia). Acta Zoologica, 63(2): 101–109. doi: 10.1111/j.1463-6395.1982.tb00765.x Julin C. 1881. Recherches sur l'organisation des Ascidies simples: 1. Sur l'hypophyse et quelques organes qui s'y rattachent, dans les genres Corella, Phallusia, Ascidia. Archives de Biologie (in German), 2(1): 59–126 Kaufman M H. 1992. The Atlas of Mouse Development. San Diego: Academic Press Kelberman D, Rizzoti K, Lovell-Badge R, et al. 2009. Genetic regulation of pituitary gland development in human and mouse. Endocrine Reviews, 30(7): 790–829. doi: 10.1210/er.2009-0008 Komai T. 1951. The homology of the “notochord” found in pterobranchs and enteropneusts. The American Naturalist, 85(823): 270–271. doi: 10.1086/281678 Lacalli T C. 1996. Frontal eye circuitry, rostral sensory pathways and brain organization in amphioxus larvae: evidence from 3D reconstructions. Philosophical Transactions of the Royal Society B: Biological Sciences, 351(1337): 243–263. doi: 10.1098/rstb.1996.0022 Lacalli T C. 2004. Sensory systems in amphioxus: a window on the ancestral chordate condition. Brain, Behavior and Evolution, 64(3): 148–162 Lacalli T C. 2008. Basic features of the ancestral chordate brain: a protochordate perspective. Brain Research Bulletin, 75(2–4): 319–323 Lacalli T. 2017. Interpreting amphioxus, and thoughts on ancestral chordate mouths and brains. International Journal of Developmental Biology, 61: 649–654. doi: 10.1387/ijdb.170105tl Lacalli T. 2018. Amphioxus neurocircuits, enhanced arousal, and the origin of vertebrate consciousness. Consciousness and Cognition, 62: 127–134. doi: 10.1016/j.concog.2018.03.006 Legros R. 1898. Développement de la cavité buccale de l’Amphioxus lanceolatus: deuxième partie. Arch Anat Microscop (in German), 2: 1–43 Li Mengyang, Gao Zhan, Ji Dongrui, et al. 2014. Functional characterization of GH-like homolog in amphioxus reveals an ancient origin of GH/GH receptor system. Endocrinology, 155(12): 4818–4830. doi: 10.1210/en.2014-1377 Li Mengyang, Jiang Chengyan, Zhang Yu, et al. 2017. Activities of amphioxus GH-like protein in osmoregulation: insight into origin of vertebrate GH family. International Journal of Endocrinology, 2017: 9538685 Nakabayashi K, Matsumi H, Bhalla A, et al. 2002. Thyrostimulin, a heterodimer of two new human glycoprotein hormone subunits, activates the thyroid-stimulating hormone receptor. The Journal of Clinical Investigation, 109(11): 1445–1452. doi: 10.1172/JCI0214340 Nozaki M, Gorbman A. 1992. The question of functional homology of Hatschek’s pit of amphioxus (Branchiostoma belcheri) and the vertebrate adenohypophysis (Endocrinology). Zoological Science, 9: 387–395 Park J I, Semyonov J, Chang Chia Lin, et al. 2005. Conservation of the heterodimeric glycoprotein hormone subunit family proteins and the LGR signaling system from nematodes to humans. Endocrine, 26(3): 267–276. doi: 10.1385/ENDO:26:3:267 Pestarino M. 1984. Immunocytochemical demonstration of prolactin-like activity in the neural gland of the ascidian Styela plicata. General and Comparative Endocrinology, 54(3): 444–449. doi: 10.1016/0016-6480(84)90160-6 Pestarino M. 1985. A pituitary-like role of the neural gland of an ascidian. General and Comparative Endocrinology, 60(2): 293–297. doi: 10.1016/0016-6480(85)90326-0 Rhodes S J, DiMattia G E, Rosenfeld M G. 1994. Transcriptional mechanisms in anterior pituitary cell differentiation. Current Opinion in Genetics & Development, 4(5): 709–717 Rizzoti K. 2015. Genetic regulation of murine pituitary development. Journal of Molecular Endocrinology, 54(2): R55–R73. doi: 10.1530/JME-14-0237 Ruppert E E. 1997. Cephalochordata (Acrania). In: Harrison F W, Ruppert E E, eds. Microscopic Anatomy of Invertebrates. Vol. 15. New York: Wiley-Liss, 349–504 Sahlin K, Olsson R. 1986. The wheel organ and Hatschek’s groove in the lancelet, Branchiostoma lanceolatum (cephalochordata). Acta Zoologica, 67(4): 201–209. doi: 10.1111/j.1463-6395.1986.tb00864.x Saint-Jeannet J P, Moody S A. 2014. Establishing the pre-placodal region and breaking it into placodes with distinct identities. Developmental Biology, 389(1): 13–27. doi: 10.1016/j.ydbio.2014.02.011 Schwind J L. 1928. The development of the hypophysis cerebri of the albino rat. American Journal of Anatomy, 41(2): 295–319. doi: 10.1002/aja.1000410206 Simmons D M, Voss J W, Ingraham H A, et al. 1990. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes & Developm, 4(5): 695–711 Singer C J. 1931. A Short History of Biology. Oxford: Clarendon Press, 7–9 Sower S A. 2015. Breaking dogma on the hypothalamic-pituitary anatomical relations in vertebrates. Endocrinology, 156(11): 3882–3884. doi: 10.1210/en.2015-1778 Sower S A, Hausken K N. 2017. A lamprey view on the origins of neuroendocrine regulation of the thyroid axis. Molecular and Cellular Endocrinology, 459: 21–27. doi: 10.1016/j.mce.2017.04.012 Sower S A, Moriyama S, Kasahara M, et al. 2006. Identification of sea lamprey GTHβ-like cDNA and its evolutionary implications. General and Comparative Endocrinology, 148(1): 22–32. doi: 10.1016/j.ygcen.2005.11.009 Sudo S, Kuwabara Y, Park J I, et al. 2005. Heterodimeric fly glycoprotein hormone-α2 (GPA2) and glycoprotein hormone-β5 (GPB5) activate fly leucine-rich repeat-containing G protein-coupled receptor-1 (DLGR1) and stimulation of human thyrotropin receptors by chimeric fly GPA2 and human GPB5. Endocrinology, 146(8): 3596–3604. doi: 10.1210/en.2005-0317 Sun Yi, Zhang Qiujin, Zhong Jing, et al. 2010. Characterization and expression of AmphiBMP3/3b gene in amphioxus Branchiostoma japonicum. Development, Growth & Differentiation, 52(2): 157–167 Tando Y, Kubokawa K. 2009a. A homolog of the vertebrate thyrostimulin glycoprotein hormone α subunit (GPA2) is expressed in amphioxus neurons. Zoological Science, 26(6): 409–414. doi: 10.2108/zsj.26.409 Tando Y, Kubokawa K. 2009b. Expression of the gene for ancestral glycoprotein hormone β subunit in the nerve cord of amphioxus. General and Comparative Endocrinology, 162(3): 329–339. doi: 10.1016/j.ygcen.2009.04.015 Tjoa L T, Welsch U. 1974. Electron microscopical observations on Kölliker’s and Hatschek’s pit and on the wheel organ in the head region of Amphioxus (Branchiostoma lanceolatum). Cell and Tissue Research, 153(2): 175–187 Treacy M N, Rosenfeld M G. 1992. Expression of a family of POU-domain protein regulatory genes during development of the central nervous system. Annual Review of Neuroscience, 15: 139–165. doi: 10.1146/annurev.ne.15.030192.001035 Veenstra J A. 2010. Neurohormones and neuropeptides encoded by the genome of Lottia gigantea, with reference to other mollusks and insects. General and Comparative Endocrinology, 167(1): 86–103. doi: 10.1016/j.ygcen.2010.02.010 Wang Peng, Liu Shousheng, Yang Qingyun, et al. 2018. Functional characterization of thyrostimulin in amphioxus suggests an ancestral origin of the TH signaling pathway. Endocrinology, 159(10): 3536–3548. doi: 10.1210/en.2018-00550 Wang Yong, Zhang Peijun, Yasui K, et al. 2002. Expression of Bblhx3, a LIM-homeobox gene, in the development of amphioxus Branchiostoma belcheri tsingtauense. Mechanisms of Development, 117(1–2): 315–319 Welsch L T, Welsch U. 1978. Histological and Elektronmicroscopical observations on the preoral ciliary groove of Saccoglossus horst (Hemichordata) and on Hatschek’s pit of Branchiostoma lanceolatum (Acrania). A contribution to the phylogenetic development of the adenohypophysis. Zoologische Jahrbücher, 100: 564–578 Zhadanov A B, Bertuzzi S, Taira M, et al. 1995. Expression pattern of the murine LIM class homeobox gene Lhx3 in subsets of neural and neuroendocrine tissues. Developmental Dynamics, 202(4): 354–364. doi: 10.1002/aja.1002020405 Zhang Shicui. 2020. Evolutionary Biology of Amphioxus (in Chinese). Beijing: Science Press Zhang Zhiyi, Zhu Yitao, Chen Dayuan. 1982. Immunocytochemical demonstration of luteinizing hormone (LH) in Hatschek’s pit of amphioxus (Branchiostoma belcheri Gray). Chinese Science Bulletin, 27(11): 1233–1234 -




 下载:
下载: