The influence of lipid-extraction on the δ13C of mesopelagic and demersal fish in the South China Sea: modification and application of lipid normalization models
-
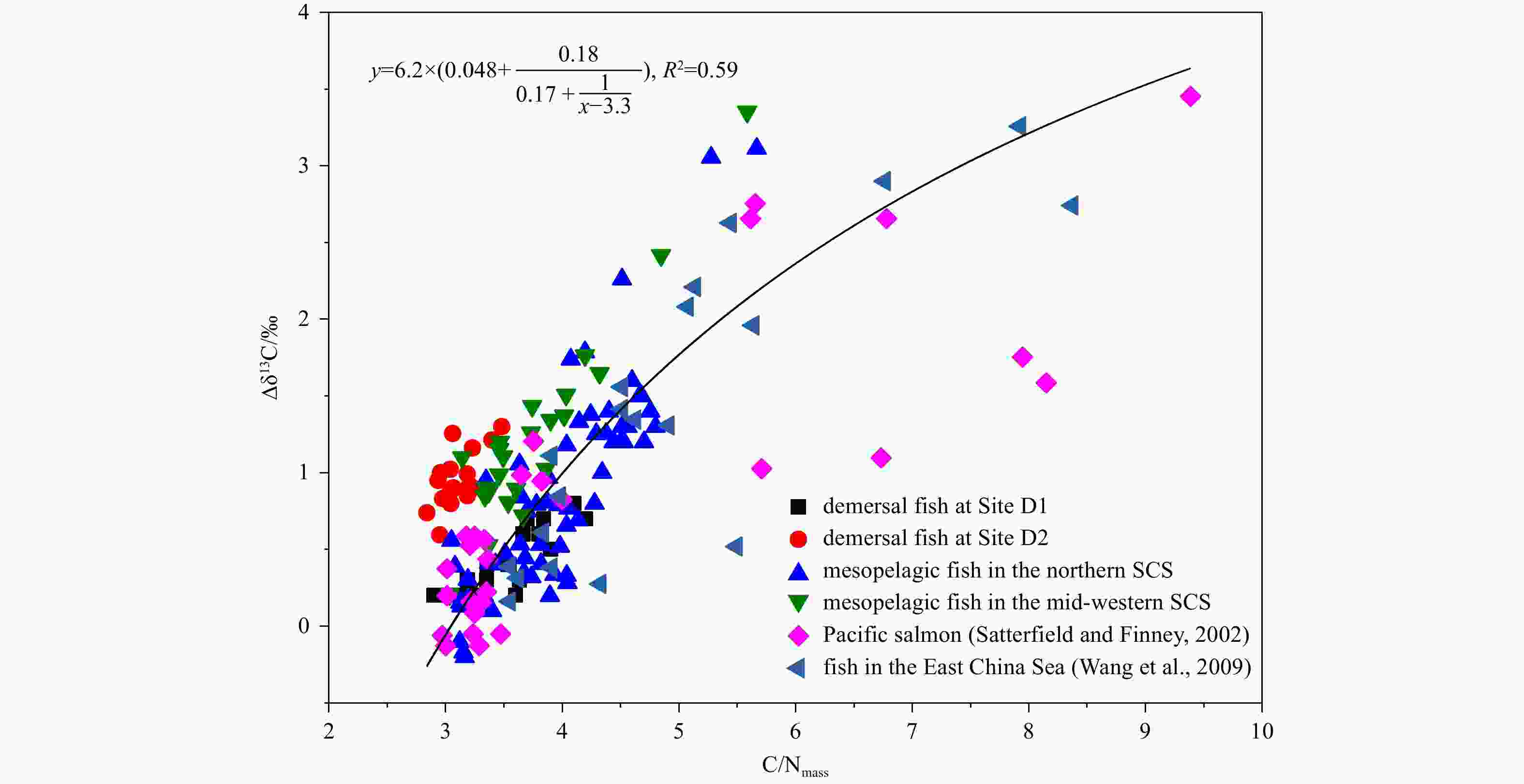
Abstract: Mesopelagic fish, the most important daily vertically migrating community in the oceans, are characterized by high lipid content which may obscure the interpretation of stable isotopes analysis. Demersal fish, which are important consumers in the food web dominated by mesopelagic fish, also have a high lipid content. Here we collected 127 fish samples from the South China Sea and evaluated the effect of lipid contents on δ13C of mesopelagic and demersal fish. In lipid-extracted mesopelagic fish, the C/N content ratio (<5.5) shows a clear correlation with Δδ13C (the offset of bulk and lipid-extracted δ13C values), especially in non-migratory and semi-migratory species; these values were less correlation in demersal fish. Based on our results, we suggest that mesopelagic and demersal fish in different regions of the South China Sea should be studied separately using appropriate correction models and less fit for the traditional model. Moreover, the C/N content ratio should be used cautiously for establishing the lipid normalization model, especially for the fish in migratory mesopelagic fish and demersal fish. Our results also reveal that mesopelagic fish across nearby regions could be analyzed together. The new models described here can be applied in future studies of mesopelagic and demersal fish in the South China Sea.
-
Key words:
- mesopelagic fish /
- demersal fish /
- lipid normalization model /
- C/N content ratio /
- lipid content /
- δ13C /
- South China Sea
-
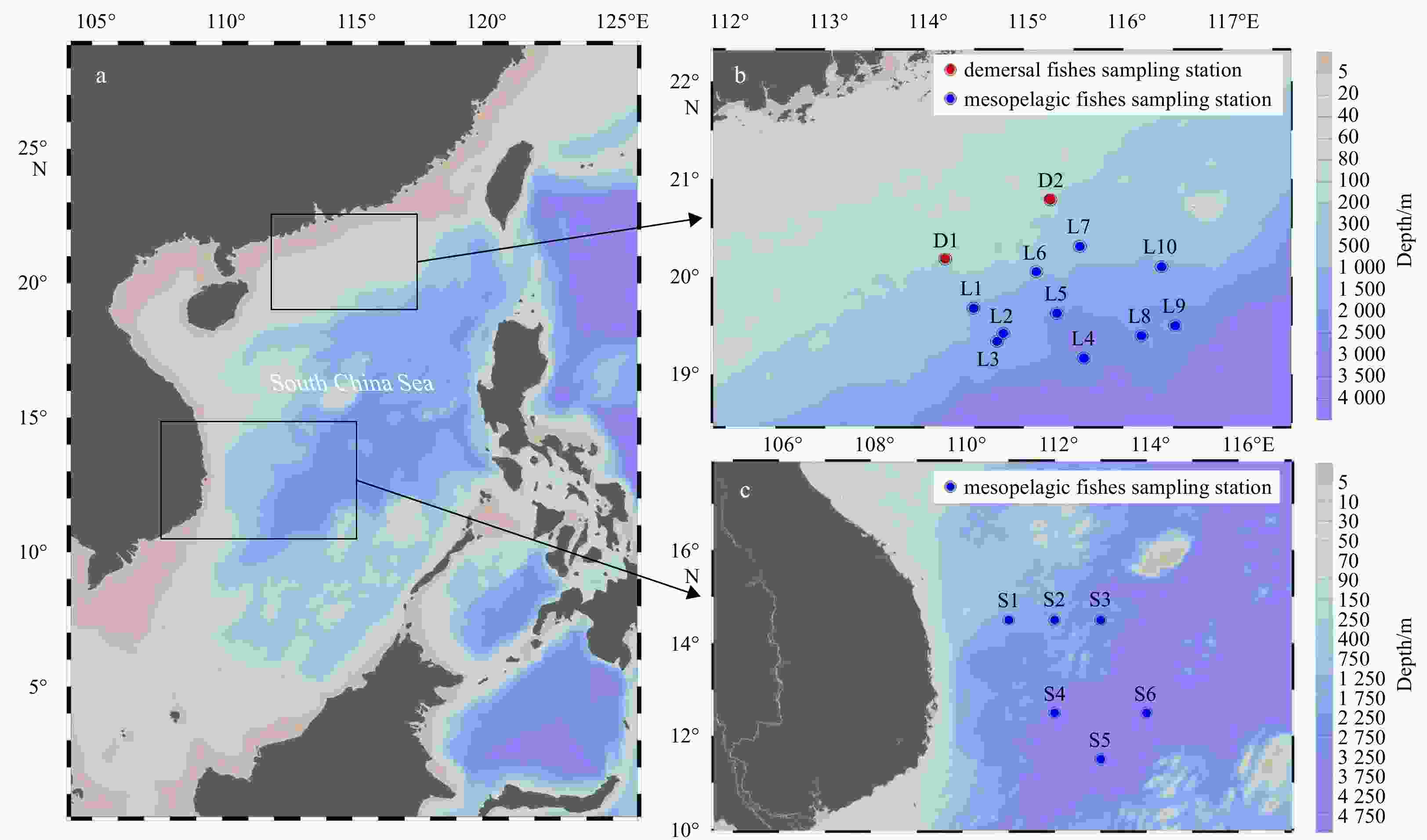
Figure 1. Map of sample collection regions in the South China Sea (SCS) (a): the north slope of the SCS (b); the mid-western SCS (c). L1–L10 represent sampling sites of mesopelagic fish on the northern slope of the SCS; D1 and D2 are sampling sites of demersal fish in the continental shelf area and shrimp farm zone of the SCS; S1–S6 represent sampling sites in the mid-western SCS.
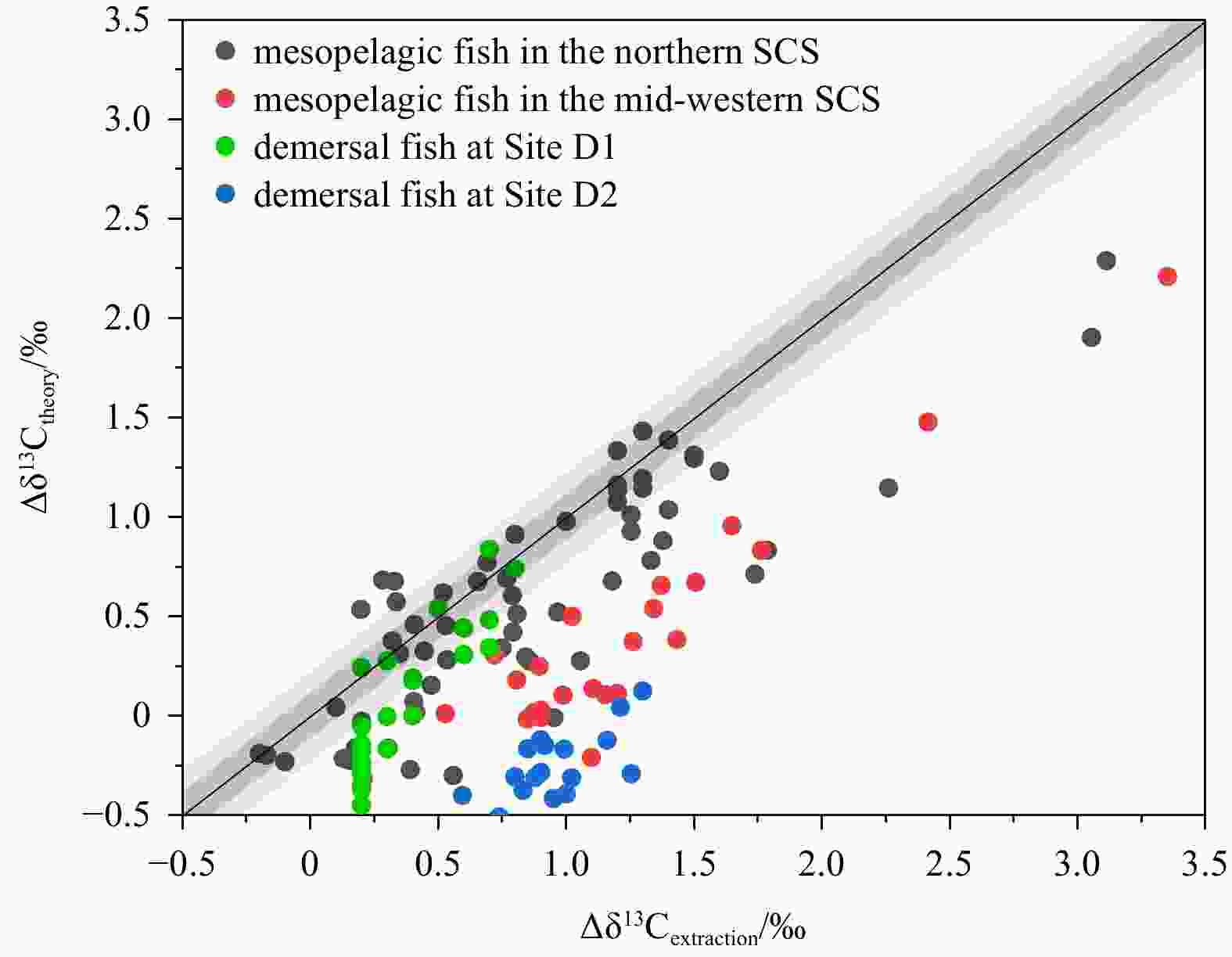
Figure 2. The difference between Δδ13Cextraction and Δδ13Ctheory in four groups of fish in the SCS. Δδ13Cextraction: the difference between bulk and lipid-extracted δ13C. Δδ13Ctheory: Δδ13C was calculated using the lipid normalization model (Post et al., 2007). Grey area: ±0.2‰ error range.
Table 1. Sample information
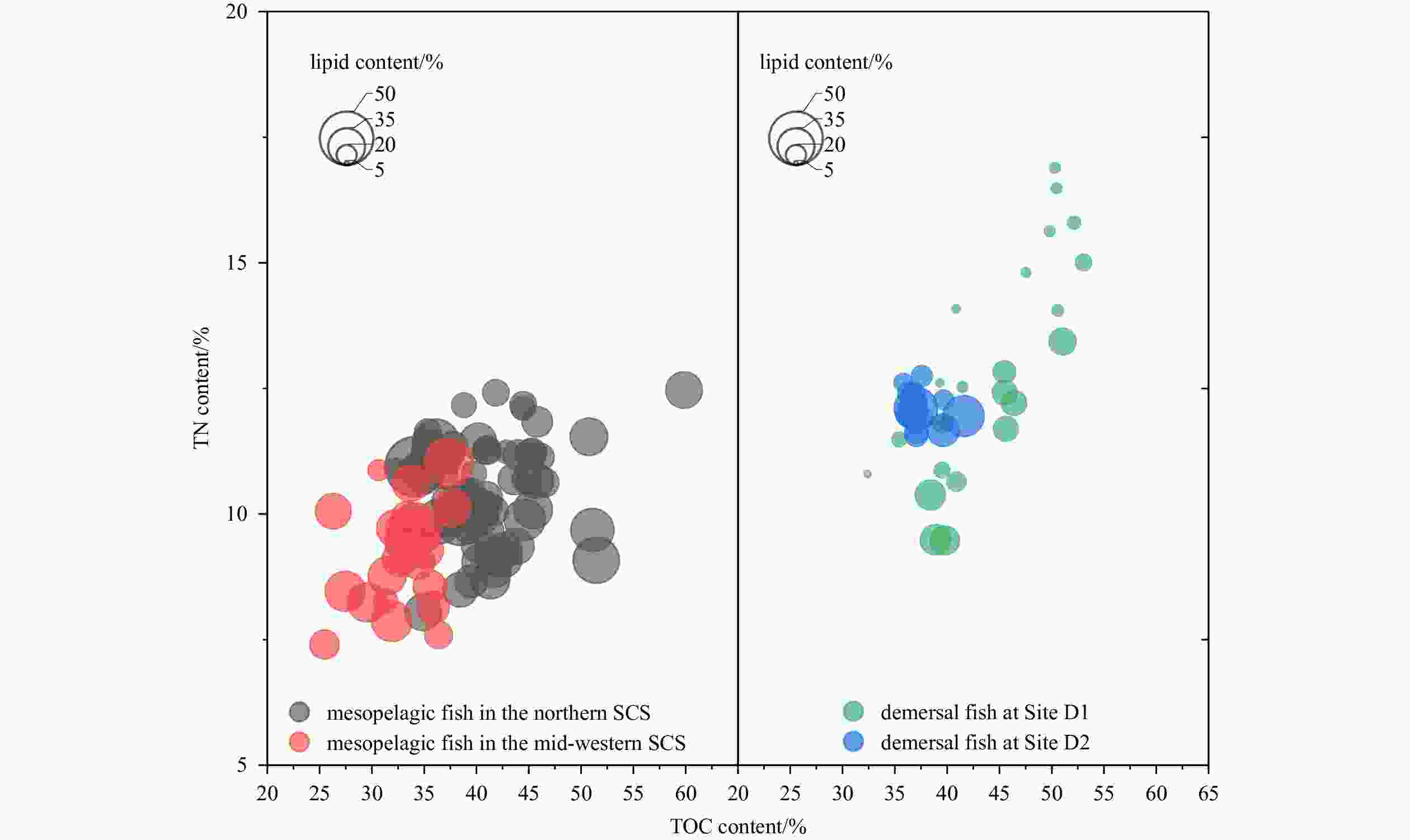
Sampling period Region Habitat Site Number Oct. 2014 north slope mesopelagic L1, L4, L5 14 Jun. 2015 north slope mesopelagic L2, L6, L8 32 Mar. 2017 north slope mesopelagic L3, L7, L9, L10 19 Oct. 2016 mid-west mesopelagic S1−S6 24 Oct. 2014 Site D1 demersal D1 24 Jun. 2015 Site D2 demersal D2 14 Table 2. Mean±SD lipid content, carbon content to nitrogen content ratios (C/Nmass), total nitrogen content (TN), total organic carbon content (TOC), and Δδ13C (the difference between bulk and lipid-extracted δ13C) in four regions of the SCS. Pearson correlation coefficient tested the correlation between lipid content and C/Nmass, C/Nmass and Δδ13C
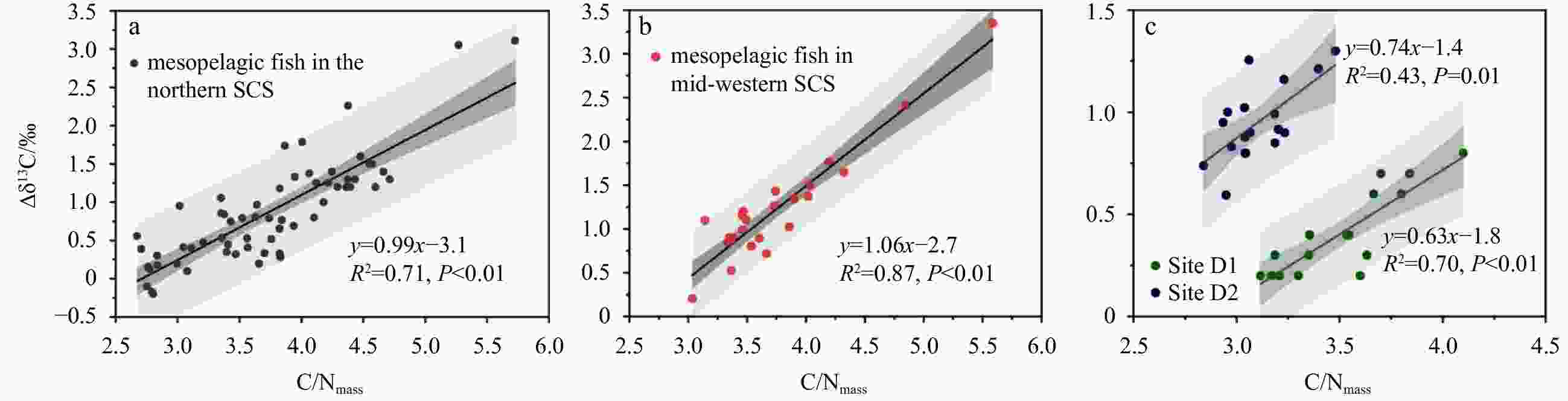
Region Lipid content/% C/Nmass TN/% TOC/% Δδ13C/‰ Lipid content vs. C/Nmass C/Nmass vs. Δδ13C P Pearson corr. P Pearson corr. North slope 30.4±6.6 3.7±0.6 10.5±1.0 40.7±5.0* 0.9±0.7* < 0.01 0.38 < 0.01 0.77 Mid-west 31.7±5.9 3.7±0.6 9.2±1.0* 32.9±3.2* 1.2±0.6 0.69 −0.09 < 0.01 0.95 Site D1 16.7±6.9 3.5±0.4 12.8±2.1 43.8±5.9 0.4±0.2 < 0.01 0.86 < 0.01 0.84 Site D2 24.5±6.8 3.1±0.2 12.1±0.3 37.5±1.6 0.9±0.2 0.06 0.53 0.03 0.57 Note: *represents a significant difference in ANOVA for different migration habits in mesopelagic fish with significant value P<0.05. -
Abrantes K G, Semmens J M, Lyle J M, et al. 2012. Normalisation models for accounting for fat content in stable isotope measurements in salmonid muscle tissue. Marine Biology, 159(1): 57–64. doi: 10.1007/s00227-011-1789-1 Bowen W D, Boness D J, Oftedal O T. 1987. Mass transfer from mother to pup and subsequent mass loss by the weaned pup in the hooded seal, Cystophora cristata. Canadian Journal of Zoology, 65(1): 1–8. doi: 10.1139/z87-001 Catul V, Gauns M, Karuppasamy P K. 2011. A review on mesopelagic fishes belonging to family Myctophidae. Reviews in Fish Biology and Fisheries, 21(3): 339–354. doi: 10.1007/s11160-010-9176-4 Chen Suzhi. 2002. Fauna Sinica: Ostichthyes (Myctophiformmes, Cetomimiformes and Osteoglossiformes) (in Chinese). Beijing: Science Press, 1–304 Chen Dagang, Zhang Meizhao. 2015. Marine Fishes of China (in Chinese). Qingdao: China Ocean University Press, 1–2152 Cherel Y, Fontaine C, Richard P, et al. 2010. Isotopic niches and trophic levels of myctophid fishes and their predators in the Southern Ocean. Limnology & Oceanography, 55(1): 324–332. doi: 10.4319/lo.2010.55.1.0324 Cloyed C S, Dacosta K P, Hodanbosi M R, et al. 2020. The effects of lipid extraction on δ13C and δ15N values and use of lipid-correction models across tissues, taxa and trophic groups. Methods in Ecology and Evolution, 11(6): 751–762. doi: 10.1111/2041-210x.13386 Cloyed C S, Eason P K. 2016. Different ecological conditions support individual specialization in closely related, ecologically similar species. Evolutionary Ecology, 30(3): 379–400. doi: 10.1007/s10682-016-9825-8 Colaço A, Giacomello E, Porteiro F, et al. 2013. Trophodynamic studies on the Condor seamount (Azores, Portugal, North Atlantic). Deep-Sea Research Part II: Topical Studies in Oceanography, 98: 178–189. doi: 10.1016/j.dsr2.2013.01.010 Cui Ying, Wu Ying, Xu Zhaoli, et al. 2015. Potential dietary influence on the stable isotopes and fatty acid composition of migratory anchovy (Coilia mystus) around the Changjiang Estuary. Journal of the Marine Biological Association of the United Kingdom, 95(1): 193–205. doi: 10.1017/S0025315414000873 De Lecea A M, De Charmoy L. 2015. Chemical lipid extraction or mathematical isotope correction models: Should mathematical models be widely applied to marine species?. Rapid Communications in Mass Spectrometry, 29(21): 2013–2025. doi: 10.1002/rcm.7310 DeNiro M J, Epstein S. 1977. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science, 197(4300): 261–263. doi: 10.1126/science.327543 Eduardo L N, Bertrand A, Mincarone M M, et al. 2021. Distribution, vertical migration, and trophic ecology of lanternfishes (Myctophidae) in the Southwestern Tropical Atlantic. Progress in Oceanography, 199: 102695. doi: 10.1016/j.pocean.2021.102695 Fagan K A, Koops M A, Arts M T, et al. 2011. Assessing the utility of C:N ratios for predicting lipid content in fishes. Canadian Journal of Fisheries and Aquatic Sciences, 68(2): 374–385. doi: 10.1139/f10-119 Fanelli E, Cartes J E, Papiol V. 2011. Food web structure of deep-sea macrozooplankton and micronekton off the Catalan slope: Insight from stable isotopes. Journal of Marine Systems, 87(1): 79–89. doi: 10.1016/j.jmarsys.2011.03.003 Flynn A J, Kloser R J. 2012. Cross-basin heterogeneity in lanternfish (family Myctophidae) assemblages and isotopic niches (δ13C and δ15N) in the southern Tasman Sea abyssal basin. Deep-Sea Research Part I: Oceanographic Research Papers, 69: 113–127. doi: 10.1016/j.dsr.2012.07.007 Folch J, Lees M, Stanley G H S. 1957. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry, 226(1): 497–509. doi: 10.1016/S0021-9258(18)64849-5 Gong Yuyan, Chen Zuozhi, Zhang Jun, et al. 2015a. Feeding habits of Diaphus chrysorhynchus from continental slope region in northern South China Sea in autumn. South China Fisheries Science, 11(5): 90–99. doi: 10.3969/j.issn.2095-0780.2015.05.011 Gong Chenglin, Wang Yingmin, Xu Shang, et al. 2015b. The northeastern South China Sea margin created by the combined action of down-slope and along-slope processes: Processes, products and implications for exploration and paleoceanography. Marine & Petroleum Geology, 64: 233–249. doi: 10.1016/j.marpetgeo.2015.01.016 Hoffman J C, Sierszen M E, Cotter A M. 2015. Fish tissue lipid-C:N relationships for correcting δ13C values and estimating lipid content in aquatic food-web studies. Rapid Communications in Mass Spectrometry, 29(21): 2069–2077. doi: 10.1002/rcm.7367 Hoffman J C, Sutton T T. 2010. Lipid correction for carbon stable isotope analysis of deep-sea fishes. Deep-Sea Research Part I: Oceanographic Research Papers, 57(8): 956–964. doi: 10.1016/j.dsr.2010.05.003 Hu Jianyu, Kawamura H, Hong Huasheng, et al. 2000. A review on the currents in the South China Sea: seasonal circulation, South China Sea warm current and Kuroshio intrusion. Journal of Oceanography, 56(6): 607–624. doi: 10.1023/A:1011117531252 Hudson J M, Steinberg D K, Sutton T T, et al. 2014. Myctophid feeding ecology and carbon transport along the northern Mid-Atlantic Ridge. Deep-Sea Research Part I: Oceanographic Research Papers, 93: 104–116. doi: 10.1016/j.dsr.2014.07.002 Irigoien X, Klevjer T A, Røstad A, et al. 2014. Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nature Communications, 5: 3271. doi: 10.1038/ncomms4271 Jin Haiwei, Xue Lijian, Pan Guoliang, et al. 2011. Feeding habits of Benthosema pterotum in the East China Sea and southern part of Yellow Sea. Marine Fisheries, 33(4): 368–377. doi: 10.3969/j.issn.1004-2490.2011.04.002 Jónasdóttir S H, Visser A W, Richardson K, et al. 2015. Seasonal copepod lipid pump promotes carbon sequestration in the deep North Atlantic. Proceedings of the National Academy of Sciences of the United States of America, 112(39): 12122–12126. doi: 10.1073/pnas.1512110112 Kong Xiaolan, Jiang Yane, Gong Yuyan, et al. 2016. A preliminary study on fishery biology of Ceratoscopelus warmingii in the central and northern South China Sea. South China Fisheries Science, 12(4): 117–124. doi: 10.3969/j.issn.2095-0780.2016.04.015 Layman C A, Arrington D A, Montaña C G, et al. 2007. Can stable isotope ratios provide for community-wide measures of trophic structure?. Ecology, 88(1): 42–48. doi: 10.1890/0012-9658(2007)88[42:CSIRPF]2.0.CO;2 Li Kaizhi, Yin Jianqiang, Huang Liangmin, et al. 2011. Distribution and abundance of thaliaceans in the northwest continental shelf of South China Sea, with response to environmental factors driven by monsoon. Continental Shelf Research, 31(9): 979–989. doi: 10.1016/j.csr.2011.03.004 Lin Mao, Lin Rongcheng. 2006. Seasonal abundance and distribution of pelagic tunicates (Chordata: Thaliacea) in the central South China Sea. Acta Oceanologica Sinica, 25(3): 148–156 Logan J M, Jardine T D, Miller T J, et al. 2008. Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. Journal of Animal Ecology, 77(4): 838–846. doi: 10.1111/j.1365-2656.2008.01394.x Masuda H, Amaoka K, Araga C, et al. 1984. The Fishes of the Japanese Archipelago. Tokyo: Tokal University Press, 1–374 McClain-Counts J P, Demopoulos A W J, Ross S W. 2017. Trophic structure of mesopelagic fishes in the Gulf of Mexico revealed by gut content and stable isotope analyses. Marine Ecology, 38(4): e12449. doi: 10.1111/maec.12449 McConnaughey T, Mcroy C P. 1979. Food-web structure and the fractionation of carbon isotopes in the Bering Sea. Marine Biology, 53(3): 257–262. doi: 10.1007/bf00952434 Molina-Valdivia V, Bustos C A, Castillo M I, et al. 2021. Oceanographic influences on the early life stages of a mesopelagic fish across the Chilean Patagonia. Progress in Oceanography, 195: 102572. doi: 10.1016/j.pocean.2021.102572 Newsome S D, Chivers S J, Kowalewski M B. 2018. The influence of lipid-extraction and long-term DMSO preservation on carbon (δ13C) and nitrogen (δ15N) isotope values in cetacean skin. Marine Mammal Science, 34(2): 277–293. doi: 10.1111/mms.12454 Newsome S D, Clementz M T, Koch P L. 2010. Using stable isotope biogeochemistry to study marine mammal ecology. Marine Mammal Science, 26(3): 509–572. doi: 10.1111/j.1748-7692.2009.00354.x Olivar M P, Bernal A, Molí B, et al. 2012. Vertical distribution, diversity and assemblages of mesopelagic fishes in the western Mediterranean. Deep-Sea Research Part I: Oceanographic Research Papers, 62: 53–69. doi: 10.1016/j.dsr.2011.12.014 Olivar M P, Bode A, López-Pérez C, et al. 2019. Trophic position of lanternfishes (Pisces: Myctophidae) of the tropical and equatorial Atlantic estimated using stable isotopes. ICES Journal of Marine Science, 76(3): 649–661. doi: 10.1093/icesjms/fsx243 Park H J, Park T H, Lee C I, et al. 2018. Ontogenetic shifts in diet and trophic position of walleye pollock, Theragra chalcogramma, in the western East Sea (Japan Sea) revealed by stable isotope and stomach content analyses. Fisheries Research, 204: 297–304. doi: 10.1016/j.fishres.2018.03.006 Patterson H K, Carmichael R H. 2016. The effect of lipid extraction on carbon and nitrogen stable isotope ratios in oyster tissues: Implications for glycogen-rich species. Rapid Communications in Mass Spectrometry, 30(24): 2594–2600. doi: 10.1002/rcm.7759 Peterson B J, Fry B. 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology & Systematics, 18(1): 293–320. doi: 10.1146/annurev.es.18.110187.001453 Post D M. 2002. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology, 83(3): 703–718. doi: 10.1890/0012-9658(2002)083[0703:Usitet]2.0.Co;2 Post D M, Layman C A, Arrington D A, et al. 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia, 152(1): 179–189. doi: 10.1007/s00442-006-0630-x Quintana-Rizzo E, Torres J J, Ross S W, et al. 2015. δ13C and δ15N in deep-living fishes and shrimps after the Deepwater Horizon oil spill, Gulf of Mexico. Marine Pollution Bulletin, 94(1–2): 241–250. doi: 10.1016/j.marpolbul.2015.02.002 Richards T M, Gipson E E, Cook A, et al. 2019. Trophic ecology of meso- and bathypelagic predatory fishes in the Gulf of Mexico. ICES Journal of Marine Science, 76(3): 662–672. doi: 10.1093/icesjms/fsy074 Saito H, Murata M. 1998. Origin of the monoene fats in the lipid of midwater fishes: relationship between the lipids of myctophids and those of their prey. Marine Ecology Progress Series, 168: 21–33. doi: 10.3354/meps168021 Satterfield F R, Finney B P. 2002. Stable isotope analysis of Pacific salmon: insight into trophic status and oceanographic conditions over the last 30 years. Progress in Oceanography, 53(2–4): 231–246, Shen Shih-chieh. 1993. Fishes of Taiwan (in Chinese). Taipei: National Taiwan University, 1–960 Stowasser G, McAllen R, Pierce G J, et al. 2009a. Trophic position of deep-sea fish—Assessment through fatty acid and stable isotope analyses. Deep-Sea Research Part I: Oceanographic Research Papers, 56(5): 812–826. doi: 10.1016/j.dsr.2008.12.016 Stowasser G, Pond D W, Collins M A. 2009b. Using fatty acid analysis to elucidate the feeding habits of Southern Ocean mesopelagic fish. Marine Biology, 156(11): 2289–2302. doi: 10.1007/s00227-009-1256-4 Su Jilan. 2004. Overview of the South China Sea circulation and its influence on the coastal physical oceanography outside the Pearl River Estuary. Continental Shelf Research, 24(16): 1745–1760. doi: 10.1016/j.csr.2004.06.005 Sun Dianrong, Chen Zheng. 2013. Review of Fishes of South China Sea: Volume One (in Chinese). Beijing: China Ocean Press, 1–606 Sutton T T. 2013. Vertical ecology of the pelagic ocean: classical patterns and new perspectives. Journal of Fish Biology, 83(6): 1508–1527. doi: 10.1111/jfb.12263 Svensson E, Freitas V, Schouten S, et al. 2014. Comparison of the stable carbon and nitrogen isotopic values of gill and white muscle tissue of fish. Journal of Experimental Marine Biology & Ecology, 457: 173–179. doi: 10.1016/j.jembe.2014.04.014 Tocher D R. 2003. Metabolism and functions of lipids and fatty acids in teleost fish. Reviews in Fisheries Science, 11(2): 107–184. doi: 10.1080/713610925 Valls M, Olivar M P, Fernández de Puelles M L, et al. 2014. Trophic structure of mesopelagic fishes in the western Mediterranean based on stable isotopes of carbon and nitrogen. Journal of Marine Systems, 138: 160–170. doi: 10.1016/j.jmarsys.2014.04.007 Wan Ruijing, Wu Ying, Huang Liang, et al. 2010. Fatty acids and stable isotopes of a marine ecosystem: study on the Japanese anchovy (Engraulis japonicus) food web in the Yellow Sea. Deep-Sea Research Part II: Topical Studies in Oceanography, 57(11–12): 1047–1057, Wang Fuqiang, Wu Ying, Chen Zuozhi, et al. 2019a. Trophic interactions of mesopelagic fishes in the South China Sea illustrated by stable isotopes and fatty acids. Frontiers in Marine Science, 5: 522. doi: 10.3389/fmars.2018.00522 Wang Fuqiang, Wu Ying, Cui Ying, et al. 2019b. δ13C and fatty acid composition of mesopelagic fishes in the South China Sea and their influence factors. Chemistry and Ecology, 35(9): 788–804. doi: 10.1080/02757540.2019.1651844 Wang Na, Wu Ying, Zhang Jing. 2009. Comparison and unification of carbon stable isotope ratios in specific aquatic biota. Communications in Nonlinear Science and Numerical Simulation, 14(5): 2502–2506. doi: 10.1016/j.cnsns.2008.05.015 Weldrick C K, Trebilco R, Swadling K M. 2019. Can lipid removal affect interpretation of resource partitioning from stable isotopes in Southern Ocean pteropods?. Rapid Communications in Mass Spectrometry, 33(6): 569–578. doi: 10.1002/rcm.8384 Yu Jie, Chen Guobao, Zhang Kui, et al. 2016. Vertical distribution of summer chlorophyll a concentration in the middle South China Sea. South China Fisheries Science, 12(4): 1–8 Yurkowski D J, Hussey N E, Semeniuk C, et al. 2015. Effects of lipid extraction and the utility of lipid normalization models on δ13C and δ15N values in Arctic marine mammal tissues. Polar Biology, 38(2): 131–143. doi: 10.1007/s00300-014-1571-1 Zhang Yi, Li Jie, Cheng Xuhua, et al. 2018. Community differentiation of bacterioplankton in the epipelagic layer in the South China Sea. Ecology and Evolution, 8(10): 4932–4948. doi: 10.1002/ece3.4064 Zhang Jun, Wang Xinliang, Jiang Yane, et al. 2019. Species composition and biomass density of mesopelagic nekton of the South China Sea continental slope. Deep-Sea Research Part II: Topical Studies in Oceanography, 167: 105–120. doi: 10.1016/j.dsr2.2018.06.008 -




 下载:
下载: