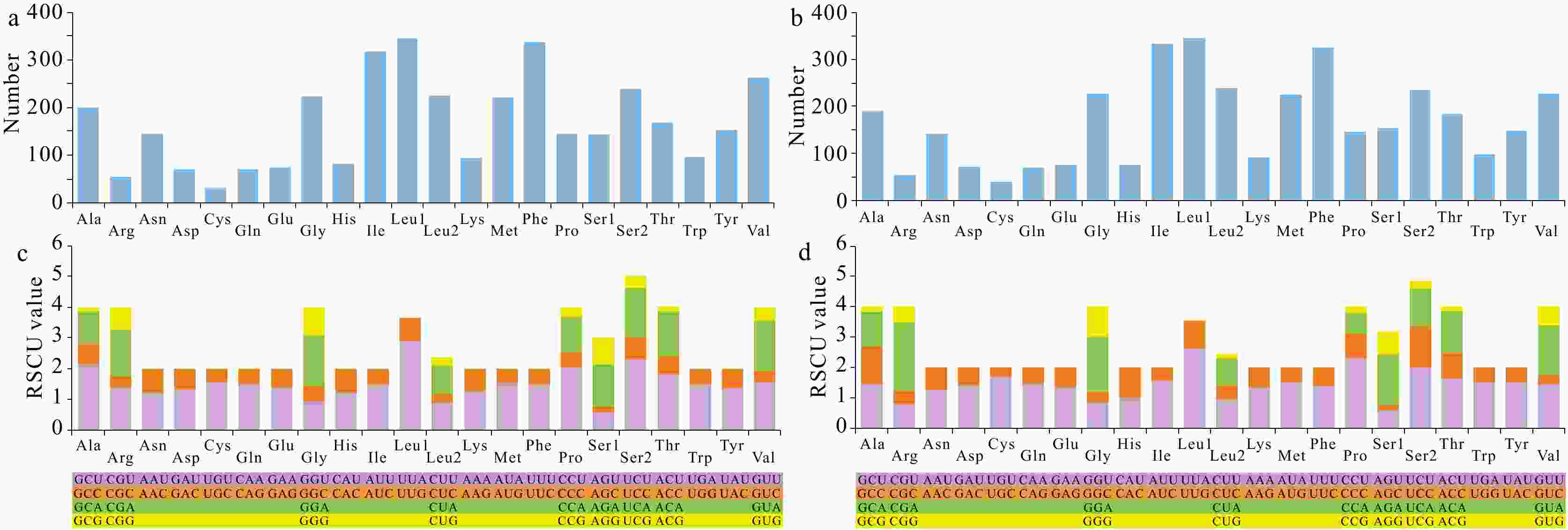
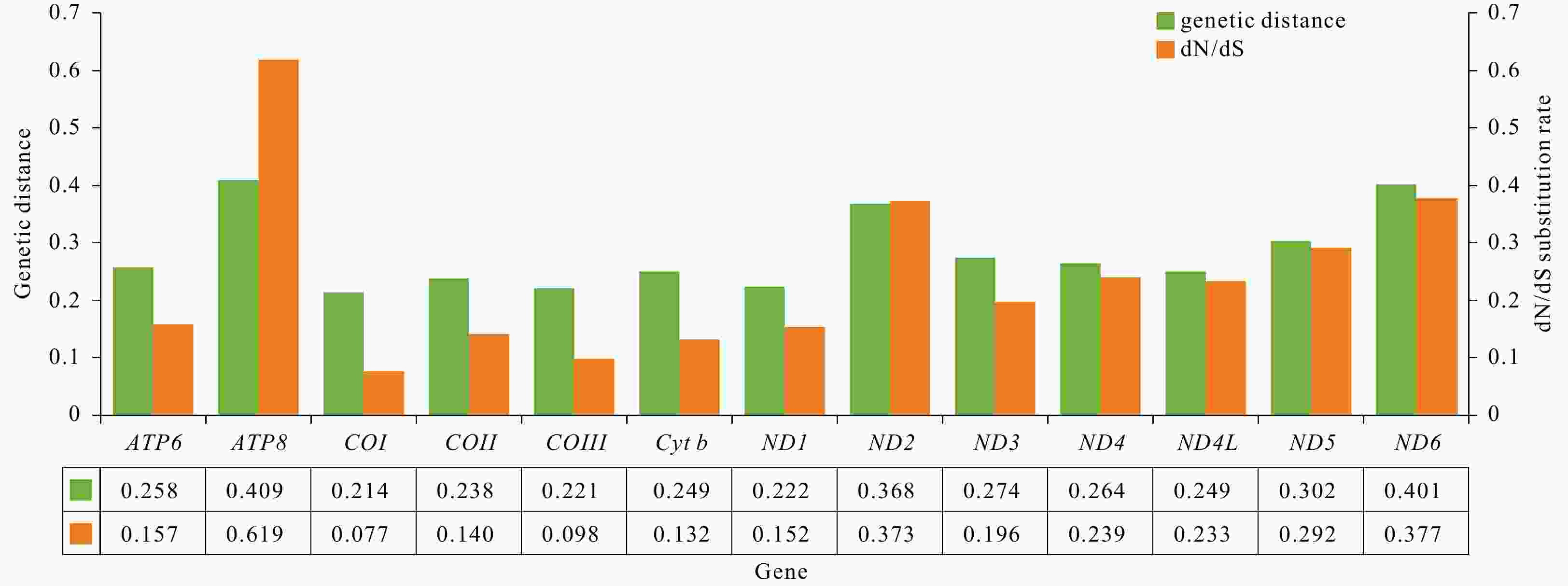
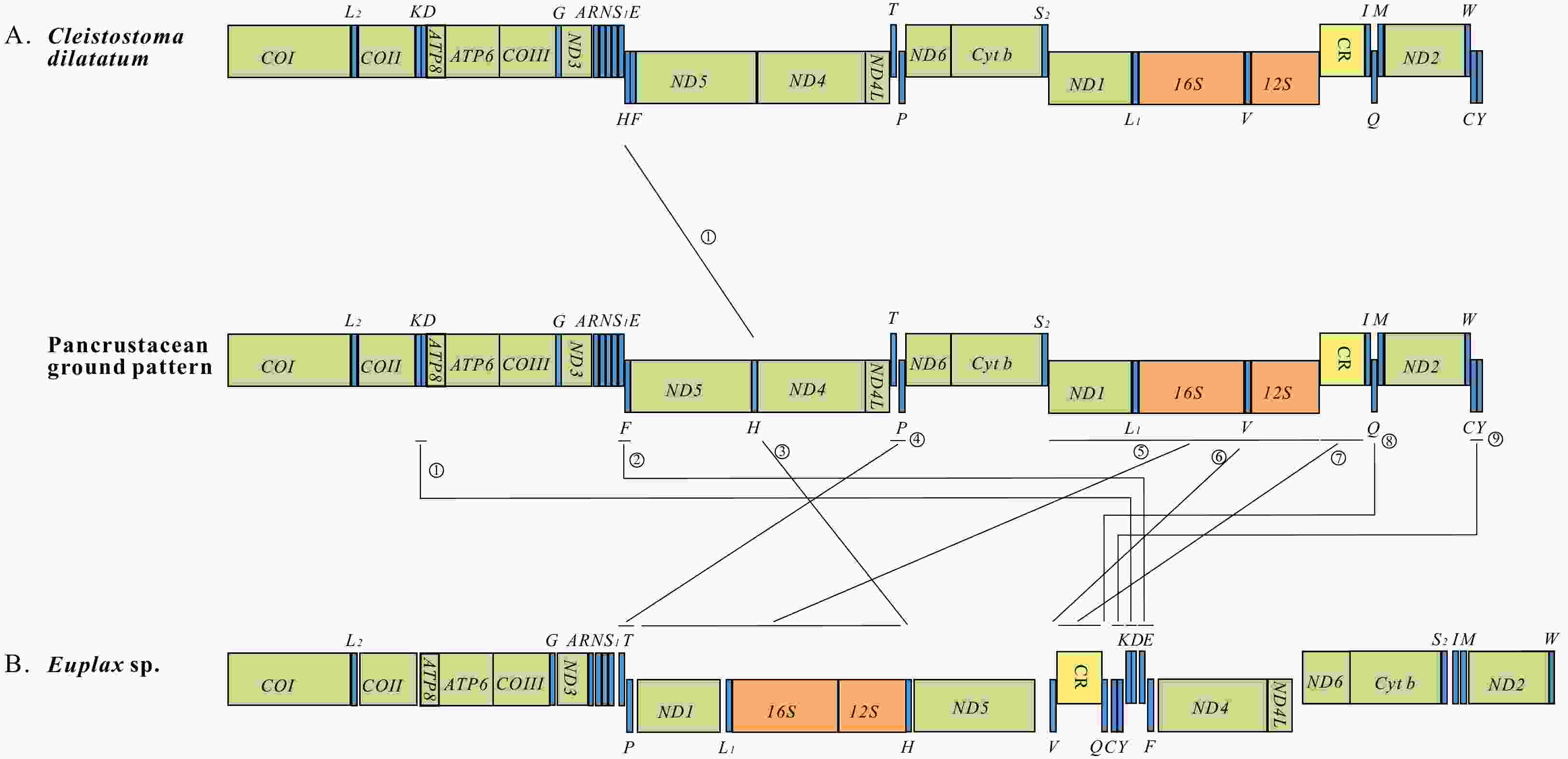
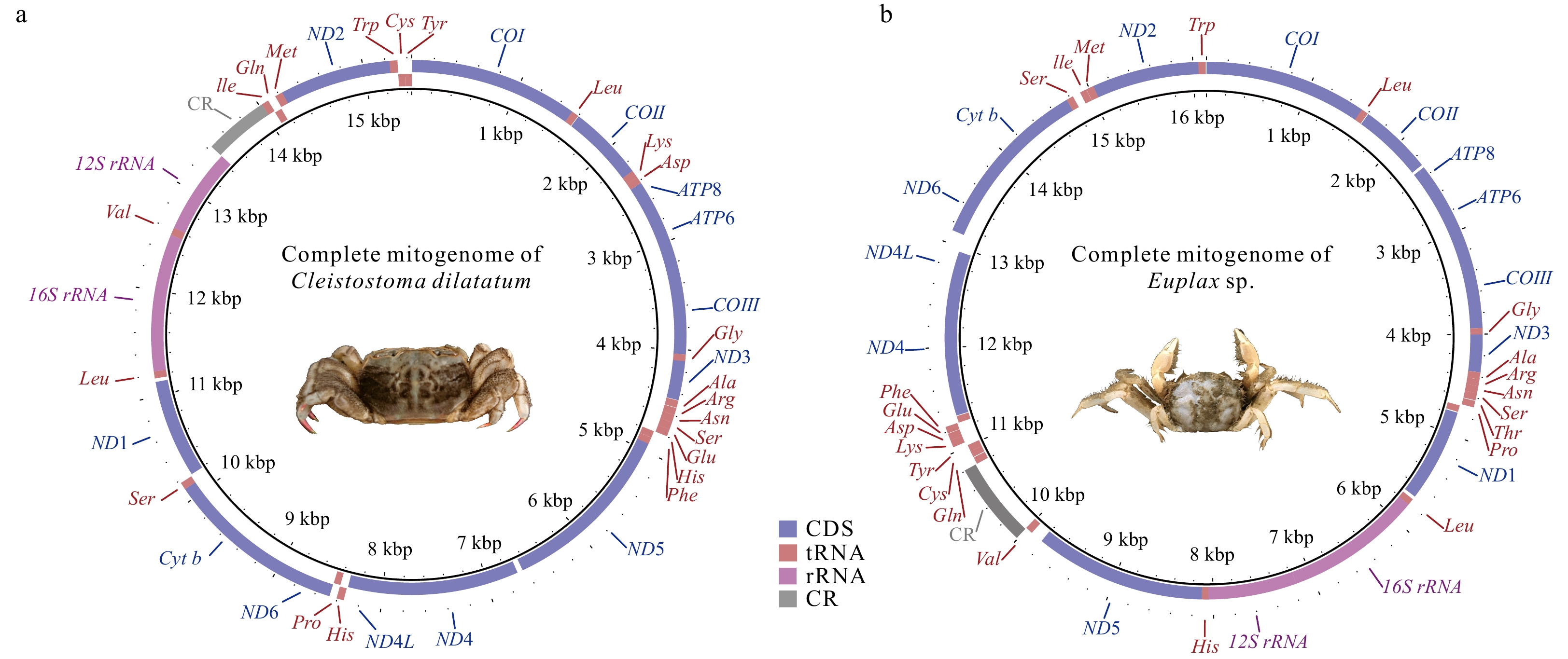
Two complete mitogenomes of Ocypodoidea (Decapoda: Brachyura), Cleistostoma dilatatum (Camptandriidae) and Euplax sp. (Macrophthalmidae) and its phylogenetic implications
-
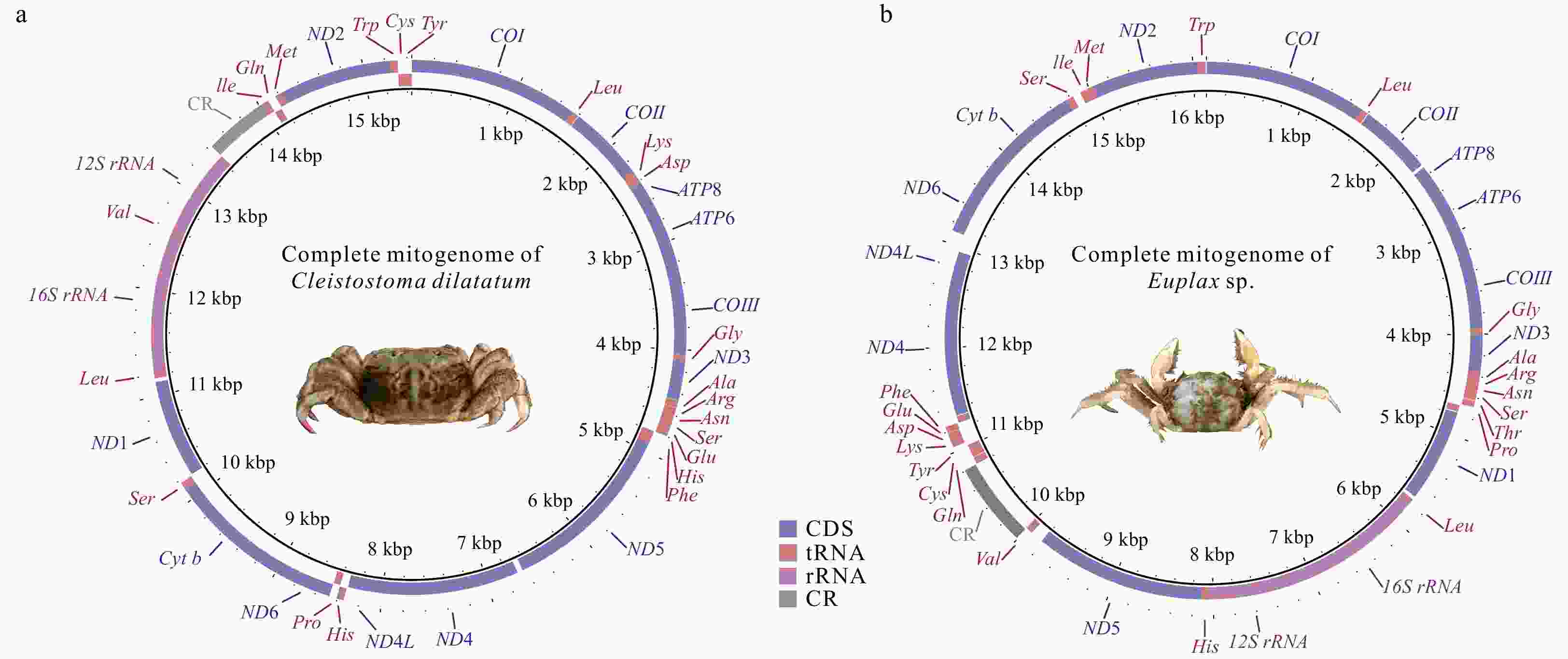
Abstract: Complete mitochondrial genomes (mitogenomes) can provide useful information for phylogenetic relationships, gene rearrangement, and molecular evolution. In the present study, two newly sequenced mitogenomes of Ocypodoidea (Cleistostoma dilatatum and Euplax sp.) were reported for the first time, which are 15 444 bp and 16 129 bp in length, respectively. Cleistostoma dilatatum is the first species in the family Camptandriidae whose complete mitogenome was sequenced. Each mitogenome contains an entire set of 37 genes and a putative control region, but their gene arrangements are largely different. Tandem duplication and random loss model is proposed to account for their gene arrangements. Comparative genomic analyses of 19 mitogenomes clustering in one branch reveal that 18 of them shared the same gene rearrangement, while that of C. dilatatum mitogenome was consistent with the ancestral gene arrangement of Brachyura. The dN/dS ratio analysis shows that all PCGs are evolving under purifying selection. Phylogenetic analyses show that all Macrophalmidae species cluster together as a group, and then form a sister clade with Camptandriidae. Moreover, the polyphyly of three superfamilies (Ocypodoidea, Eriphioidea, and Grapsoidea) is reconfirmed. These findings help to confirm the phylogenetic position of Camptandriidae, as well as provide new insights into the phylogeny of Brachyura.
-
Key words:
- Camptandriidae /
- Macrophthalmidae /
- mitogenome /
- gene rearrangement /
- phylogenetic analysis
-
Figure 4. Multiple genome alignments of 19 mitogenomes. The mitogenome of Eriocheir sinensis at the top as the reference genome. All genomes are started from the COI gene. The number at the top of each genome shows nucleotide positions. Within each of the alignments, local collinear blocks are represented by blocks of the same color connected by lines.
Figure 6. Inferred intermediate steps between the ancestral gene arrangement of crustaceans and two newly sequenced mitogenomes. The ancestral gene arrangement of crustaceans (A); the results of one tandem duplication and random loss (TDRL) event, the ancestral gene arrangement in Brachyuran mitogenome, and the final gene arrangement in Cleistostoma dilatatum mitogenome (B); the results of two TDRL events, the ancestral gene arrangement in Varunidae and Macrophthalmidae mitogenomes, and the final gene arrangement in Euplax sp. mitogenome (C). The duplicated gene block is underlined and the lost genes are labeled with gray.
Figure 7. Phylogenetic tree of brachyuran species inferred from the nucleotide sequences of 13 PCGs based on maximum likelihood (ML) and Bayesian inference (BI) analyses. The node marked with a solid circle indicates 100 ML bootstrap support and 100% BI posterior probability. The numbers after the species name are the GenBank accession number.
Table 1. Features of the mitochondrial genome of Cleistostoma dilatatum
Gene Position Length/bp Amino acid Start/Stop codon Anticodon Intergenic region Strand From To COI 1 bp 1534 bp 1534 511 ATG/T – 0 H Leu (L2) 1535 bp 1599 bp 65 – – TAA 6 H COII 1606 bp 2293 bp 688 229 ATG/T 0 H Lys (K) 2294 bp 2363 bp 70 – – TTT 0 H Asp (D) 2364 bp 2424 bp 61 – – GTC 1 H ATP8 2426 bp 2584 bp 159 52 ATG/TAA – –4 H ATP6 2581 bp 3252 bp 672 223 ATA/TAA – –1 H COIII 3252 bp 4041 bp 790 263 ATG/T – 0 H Gly (G) 4042 bp 4105 bp 64 – – TCC –3 H ND3 4103 bp 4456 bp 354 117 ATT/TAA – 4 H Ala (A) 4461 bp 4525 bp 65 – – TGC 4 H Arg (R) 4530 bp 4593 bp 64 – – TCG 0 H Asn (N) 4594 bp 4662 bp 69 – – GTT 0 H Ser (S1) 4663 bp 4729 bp 67 – – TCT 0 H Glu (E) 4730 bp 4795 bp 66 – – TTC 2 H His (H) 4798 bp 4862 bp 65 – – GTG 1 L Phe (F) 4864 bp 4928 bp 65 – – GAA –1 L ND5 4928 bp 6643 bp 1716 571 ATT/TAA – 53 L ND4 6697 bp 8034 bp 1338 445 ATG/TAA – –7 L ND4L 8028 bp 8330 bp 303 100 ATG/TAA – 9 L Thr (T) 8340 bp 8405 bp 66 – – TGT 0 H Pro (P) 8406 bp 8470 bp 65 – – TGG 2 L ND6 8473 bp 8976 bp 504 167 ATT/TAA – –1 H Cyt b 8976 bp 10 110 bp 1135 378 ATG/T – 0 H Ser (S2) 10 111 bp 10 177 bp 67 – – TGA 15 H ND1 10 193 bp 11 131 bp 939 312 ATA/TAA – 34 L Leu (L1) 11 166 bp 11 232 bp 67 – – TAG 0 L 16S 11 233 bp 12 546 bp 1314 – – – 0 L Val (V) 12 547 bp 12 619 bp 73 – – TAC 0 L 12S 12 620 bp 13 435 bp 816 – – – 0 L CR 13 436 bp 14 024 bp 589 – – – 0 H Ile (I) 14 025 bp 14 090 bp 66 – – GAT –3 H Gln (Q) 14 088 bp 14 156 bp 69 – – TTG 8 L Met (M) 14 165 bp 14 234 bp 70 – – CAT 0 H ND2 14 235 bp 15 245 bp 1011 336 ATT/TAG – –2 H Trp (W) 15 244 bp 15 315 bp 72 – – TCA 1 H Cys (C) 15 317 bp 15 380 bp 64 – – GCA 0 L Tyr (Y) 15 381 bp 15 444 bp 64 – – GTA –1 L Note: – represents no data. Table 2. Features of the mitochondrial genome of Euplax sp.
Gene Position Length/bp Amino acid Start/Stop codon Anticodon Intergenic region Strand From To COI 1 bp 1539 bp 1539 512 ATG/TAA – –5 H Leu (L2) 1535 bp 1600 bp 66 – – TAA 8 H COII 1609 bp 2296 bp 688 229 ATG/T – 28 H ATP8 2325 bp 2486 bp 162 53 ATT/TAA – –4 H ATP6 2 483 bp 3154 bp 672 223 ATA/TAA – –1 H COIII 3154 bp 3943 bp 790 263 ATG/T – 0 H Gly (G) 3 944 bp 4006 bp 63 – – TCC –3 H ND3 4004 bp 4357 bp 354 117 ATA/TAA – 1 H Ala (A) 4359 bp 4422 bp 64 – – TGC 1 H Arg (R) 4424 bp 4487 bp 64 – – TCG 0 H Asn (N) 4488 bp 4554 bp 67 – – GTT 0 H Ser (S1) 4555 bp 4621 bp 67 – – TCT 6 H Thr (T) 4 628 bp 4689 bp 62 – – TGT 16 H Pro (P) 4 706 bp 4770 bp 65 – – TGG 10 L ND1 4781 bp 5707 bp 927 308 ATA/TAG – 33 L Leu (L1) 5741 bp 5807 bp 67 – – TAG 0 L 16S 5808 bp 7170 bp 1363 – – – 0 L 12S 7171 bp 8048 bp 878 – – – 0 L His (H) 8 049 bp 8113 bp 65 – – GTG –1 L ND5 8113 bp 9813 bp 1701 566 ATG/TAA – 125 L Val (V) 9939 bp 10 011 bp 73 – – TAG 0 L CR 10 012 bp 10 806 bp 795 – – – 0 H Gln (Q) 10 807 bp 10 875 bp 69 – – TTG 7 L Cys (C) 10 883 bp 10 944 bp 62 – – GCA 0 L Tyr (Y) 10 945 bp 11 010 bp 66 – – GTA 37 L Lys (K) 11 048 bp 11 116 bp 69 – – TTT 0 H Asp (D) 11 117 bp 11 182 bp 66 – – GTC 4 H Glu (E) 11 187 bp 11 249 bp 63 – – TTC –1 H Phe (F) 11 249 bp 11 314 bp 66 – – GAA 7 L ND4 11 322 bp 12 659 bp 1338 445 ATG/TAA – –7 L ND4L 12 653 bp 12 955 bp 303 100 ATG/TAA – 169 L ND6 13 125 bp 13 649 bp 525 174 ATT/TAA – –20 H Cyt b 13 630 bp 14 764 bp 1135 378 ATG/T – 0 H Ser (S2) 14 765 bp 14 830 bp 66 – – TGA 76 H Ile (I) 14 907 bp 14 971 bp 65 – – GAT 2 H Met (M) 14 974 bp 15 042 bp 69 – – CAT 0 H ND2 15 043 bp 16 053 bp 1011 336 ATG/TAG – –2 H Trp (W) 16 052 bp 16 121 bp 70 – – TCA 7 H Note: – represents no data. -
Akasaki T, Nikaido M, Tsuchiya K, et al. 2006. Extensive mitochondrial gene arrangements in coleoid Cephalopoda and their phylogenetic implications. Molecular Phylogenetics and Evolution, 38(3): 648–658. doi: 10.1016/j.ympev.2005.10.018 Alikhan N F, Petty N K, Ben Zakour N L, et al. 2011. BLAST ring image generator (BRIG): simple prokaryote genome comparisons. BMC Genomics, 12(1): 402. doi: 10.1186/1471-2164-12-402 Bernt M, Donath A, Jühling F, et al. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Molecular Phylogenetics and Evolution, 69(2): 313–319. doi: 10.1016/j.ympev.2012.08.023 Boore J L. 1999. Animal mitochondrial genomes. Nucleic Acids Research, 27(8): 1767–1780. doi: 10.1093/nar/27.8.1767 Camargo T R, Wolf M R, Mantelatto F L, et al. 2020. Ultrastructure of spermatozoa of members of Calappidae, Aethridae and Menippidae and discussion of their phylogenetic placement. Acta Zoologica, 101(1): 89–100. doi: 10.1111/azo.12273 Chen Jianqin, Xing Yuhui, Yao Wenjia, et al. 2018. Characterization of four new mitogenomes from Ocypodoidea & Grapsoidea, and phylomitogenomic insights into thoracotreme evolution. Gene, 675: 27–35. doi: 10.1016/j.gene.2018.06.088 Chen Jianqin, Xing Yuhui, Yao Wenjia, et al. 2019. Phylomitogenomics reconfirm the phylogenetic position of the genus Metaplax inferred from the two grapsid crabs (Decapoda: Brachyura: Grapsoidea). PLoS ONE, 14(1): e0210763. doi: 10.1371/journal.pone.0210763 Cheryl T G S. 1997. A Revision of the Family Camptandriidae (Crustacea: Decapoda: Brachyura) with Notes on their Ecology. Singapore: National University of Singapore Darling A C E, Mau B, Blattner F R, et al. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Research, 14(7): 1394–1403. doi: 10.1101/gr.2289704 Davie P J F. 2002. Crustacea: Malacostraca: Eucarida (Part 2): Decapoda–Anomura, Brachyura. In: Wells A, Houston W W K, eds. Zoological Catalogues of Australia, 19.3B. Melbourne: CSIRO Publishing, 641 Davie P J F, Guinot D, Ng P K L. 2015a. Phylogeny of Brachyura. In: Castro P, Davie P J F, Guinot D, et al., eds. Treatise on Zoology—Anatomy, Taxonomy, Biology—The Crustacea, Complementary to the Volumes Translated from the French of the Traité de Zoologie, 9(C) (I), Decapoda: Brachyura (Part 2). Leiden: Brill, 921–979 Davie P J F, Guinot D, Ng P K L. 2015b. Systematics and classification of Brachyura. In: Castro P, Davie P J F, Guinot D, et al., eds. Treatise on Zoology—Anatomy, Taxonomy, Biology—The Crustacea, Complementary to the Volumes Translated from the French of the Traité de Zoologie, 9(C) (I), Decapoda: Brachyura (Part 2). Leiden: Brill, 1049–1130 Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Research, 45(4): e18 Gong Li, Lu Xinting, Luo Hairong, et al. 2020. Novel gene rearrangement pattern in Cynoglossus melampetalus mitochondrial genome: New gene order in genus Cynoglossus (Pleuronectiformes: Cynoglossidae). International Journal of Biological Macromolecules, 149: 1232–1240. doi: 10.1016/j.ijbiomac.2020.02.017 Jiang Lihua, Kang Lishen, Wu Changwen, et al. 2018. A comprehensive description and evolutionary analysis of 9 Loliginidae mitochondrial genomes. Hydrobiologia, 808(1): 115–124. doi: 10.1007/s10750-017-3377-y Jones D A, Clayton D. 1983. The systematics and ecology of crabs belonging to the genera Cleistostoma De Haan and Paracleistostoma De Man on Kuwait Mudflats. Crustaceana, 45(2): 183–199. doi: 10.1163/156854083X00613 Kalyaanamoorthy S, Minh B Q, Wong T K F, et al. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods, 14(6): 587–589. doi: 10.1038/nmeth.4285 Katoh K, Misawa K, Kuma K I, et al. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30(14): 3059–3066. doi: 10.1093/nar/gkf436 Kitaura J, Wada K, Nishida M. 1998. Molecular phylogeny and evolution of unique mud-using territorial behavior in ocypodid crabs (Crustacea: Brachyura: Ocypodidae). Molecular Biology and Evolution, 15(6): 626–637. doi: 10.1093/oxfordjournals.molbev.a025966 Kitaura J, Wada K, Nishida M. 2002. Molecular phylogeny of grapsoid and ocypodoid crabs with special reference to the genera Metaplax and Macrophthalmus. Journal of Crustacean Biology, 22(3): 682–693. doi: 10.1163/20021975-99990281 Kumar S, Stecher G, Li M, et al. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6): 1547–1549. doi: 10.1093/molbev/msy096 Lavrov D V, Boore J L, Brown W M. 2002. Complete mtDNA sequences of two millipedes suggest a new model for mitochondrial gene rearrangements: duplication and nonrandom loss. Molecular Biology and Evolution, 19(2): 163–169. doi: 10.1093/oxfordjournals.molbev.a004068 Li Gong, Shi Wei, Si Lizhen, et al. 2013. Rearrangement of mitochondrial genome in fishes. Zoological Research, 34(6): 666–673 Li Yuetian, Xin Zhaozhe, Tang Yingyu, et al. 2020. Comparative mitochondrial genome analyses of sesarmid and other brachyuran crabs reveal gene rearrangements and phylogeny. Frontiers in Genetics, 11: 536640. doi: 10.3389/fgene.2020.536640 Liu Qiuning, Xin Zhaozhe, Zhu Xiaoyu, et al. 2017. A transfer RNA gene rearrangement in the lepidopteran mitochondrial genome. Biochemical and Biophysical Research Communications, 489(2): 149–154. doi: 10.1016/j.bbrc.2017.05.115 Lowe T M, Chan P P. 2016. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Research, 44(W1): W54–W57. doi: 10.1093/nar/gkw413 Lu Xinting, Gong Li, Zhang Ying, et al. 2020. The complete mitochondrial genome of Calappa bilineata: The first representative from the family Calappidae and its phylogenetic position within Brachyura. Genomics, 112(3): 2516–2523. doi: 10.1016/j.ygeno.2020.02.003 Ma Kayan, Qin Jing, Lin Chia-Wei, et al. 2019. Phylogenomic analyses of brachyuran crabs support early divergence of primary freshwater crabs. Molecular Phylogenetics and Evolution, 135: 62–66. doi: 10.1016/j.ympev.2019.02.001 Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet Journal, 17(1): 10–12. doi: 10.14806/ej.17.1.200 Mclay C L, Kitaura J, Wada K. 2010. Behavioural and molecular evidence for the systematic position of Macrophthalmus (Hemiplax) hirtipes Hombron & Jacquinot, 1846, with comments on macrophthalmine subgenera (Decapoda, Brachyura, Macrophthalmidae). In: Fransen C H J M, De Grave S, Ng P K L, eds. Studies on Malacostraca: Lipke Bijdeley Holthuis Memorial Volume. Leiden: Brill, 483–503 Mendoza J C E, Ng P K L. 2007. Macrophthalmus (Euplax) H. Milne Edwards, 1852, a Valid Subgenus of Ocypodoid Crab (Decapoda: Brachyura: Macrophthalmidae), with Description of a New Species from the Philippines. Journal of Crustacean Biology, 27(4): 670–680. doi: 10.1651/S-2779.1 Miura T, Kawane M, Wada K. 2007. A new species of Deiratonotus (Crustacea: Brachyura: Camptandriidae) found in the Kumanoe River Estuary, Kyushu, Japan. Zoological Science, 24(10): 1045–1050. doi: 10.2108/zsj.24.1045 Moritz C, Brown W M. 1987. Tandem duplications in animal mitochondrial DNAs: variation in incidence and gene content among lizards. Proceedings of the National Academy of Sciences of the United States of America, 84(20): 7183–7187. doi: 10.1073/pnas.84.20.7183 Moritz C, Dowling T E, Brown W M. 1987. Evolution of animal mitochondrial DNA: relevance for population biology and systematics. Annual Review of Ecology and Systematics, 18: 269–292. doi: 10.1146/annurev.es.18.110187.001413 Naderloo R. 2017a. Family Camptandriidae Stimpson, 1858. In: Naderloo R, ed. Atlas of Crabs of the Persian Gulf. Cham: Springer, 369–378 Naderloo R. 2017b. Family Macrophthalmidae Dana, 1851 (Sentinel Crabs). In: Naderloo R, ed. Atlas of Crabs of the Persian Gulf. Cham: Springer, 387–403 Naruse T, Chung A Y C, Tangah J. 2015. Description of a new genus and a new species of the family Camptandriidae Stimpson, 1858 (Crustacea: Decapoda: Brachyura) from Lower Kinabatangan-Segama Wetlands, Sabah, Malaysia. Raffles Bulletin of Zoology, 63: 327–333 Ng P K L, Guinot D, Davie P J F. 2008. Systema Brachyurorum: Part I. An annotated checklist of extant brachyuran crabs of the world. The Raffles Bulletin of Zoology, 17(S1): 1–286 Nguyen L T, Schmidt H A, Von Haeseler A, et al. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32(1): 268–274. doi: 10.1093/molbev/msu300 Ojala D, Montoya J, Attardi G. 1981. tRNA punctuation model of RNA processing in human mitochondria. Nature, 290(5806): 470–474. doi: 10.1038/290470a0 Perna N T, Kocher T D. 1995. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. Journal of Molecular Evolution, 41(3): 353–358. doi: 10.1007/BF01215182 Poulton J, Deadman M E, Bindoff L, et al. 1993. Families of mtDNA re-arrangements can be detected in patients with mtDNA deletions: duplications may be a transient intermediate form. Human Molecular Genetics, 2(1): 23–30. doi: 10.1093/hmg/2.1.23 Ronquist F, Teslenko M, Van Der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61(3): 539–542. doi: 10.1093/sysbio/sys029 Ruan Huiting, Li Min, Li Zhenhai, et al. 2020. Comparative analysis of complete mitochondrial genomes of three Gerres Fishes (Perciformes: Gerreidae) and primary exploration of their evolution history. International Journal of Molecular Sciences, 21(5): 1874. doi: 10.3390/ijms21051874 Schweitzer C E, Feldmann R M. 2010. The oldest brachyura (Decapoda: Homolodromioidea: Glaessneropsoidea) known to date (Jurassic). Journal of Crustacean Biology, 30(2): 251–256. doi: 10.1651/09-3231.1 Shi Wei, Miao Xianguang, Kong Xiaoyu. 2014. A novel model of double replications and random loss accounts for rearrangements in the Mitogenome of Samariscus latus (Teleostei: Pleuronectiformes). BMC Genomics, 15(1): 352. doi: 10.1186/1471-2164-15-352 Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology, 56(4): 564–577. doi: 10.1080/10635150701472164 Tan Mun Hua, Gan Hanming, Lee Yinpeng, et al. 2018. ORDER within the chaos: Insights into phylogenetic relationships within the Anomura (Crustacea: Decapoda) from mitochondrial sequences and gene order rearrangements. Molecular Phylogenetics and Evolution, 127: 320–331. doi: 10.1016/j.ympev.2018.05.015 Trivedi J N, Trivedi D J, Vachharajani K D. 2017. Range extension of brachyuran crabs of the family Camptandriidae Stimpson, 1858 (Crustacea: Decapoda: Brachyura) in Indian waters. Check List, 13(3): 2145. doi: 10.15560/13.3.2145 Wang Qi, Tang Dan, Guo Huayun, et al. 2020. Comparative mitochondrial genomic analysis of Macrophthalmus pacificus and insights into the phylogeny of the Ocypodoidea & Grapsoidea. Genomics, 112(1): 82–91. doi: 10.1016/j.ygeno.2019.12.012 Wu Xiangyun, Li Xiaoling, Li Lu, et al. 2012. New features of Asian Crassostrea oyster mitochondrial genomes: a novel alloacceptor tRNA gene recruitment and two novel ORFs. Gene, 507(2): 112–118. doi: 10.1016/j.gene.2012.07.032 Xie Guanglong, Köhler F, Huang Xiaochen, et al. 2019. A novel gene arrangement among the Stylommatophora by the complete mitochondrial genome of the terrestrial slug Meghimatium bilineatum (Gastropoda, Arionoidea). Molecular Phylogenetics and Evolution, 135: 177–184. doi: 10.1016/j.ympev.2019.03.002 Yang Ziheng. 2006. Computational Molecular Evolution. Oxford: Oxford University Press, 259–292 Yang Mei, Dong Dong, Li Xinzheng. 2021. The complete mitogenome of Phymorhynchus sp. (Neogastropoda, Conoidea, Raphitomidae) provides insights into the deep-sea adaptive evolution of Conoidea. Ecology and Evolution, 11(12): 7518–7531. doi: 10.1002/ece3.7582 Yuan Yang, Li Qi, Yu Hong, et al. 2012. The complete mitochondrial genomes of six heterodont bivalves (Tellinoidea and Solenoidea): variable gene arrangements and phylogenetic implications. PLoS ONE, 7(2): e32353. doi: 10.1371/journal.pone.0032353 Zhang Ying, Gao Yan, Gong Li, et al. 2021a. Mitochondrial genome of Episesarma lafondii (Brachyura: Sesarmidae) and comparison with other sesarmid crabs. Journal of Ocean University of China, 20(6): 1545–1556. doi: 10.1007/s11802-021-4779-z Zhang Dong, Gao Fangluan, Jakovlić I, et al. 2020a. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources, 20(1): 348–355. doi: 10.1111/1755-0998.13096 Zhang Ying, Gong Li, Lu Xinting, et al. 2020b. Gene rearrangements in the mitochondrial genome of Chiromantes eulimene (Brachyura: Sesarmidae) and phylogenetic implications for Brachyura. International Journal of Biological Macromolecules, 162: 704–714. doi: 10.1016/j.ijbiomac.2020.06.196 Zhang Ying, Meng Lei, Wei Liming, et al. 2021b. Different gene rearrangements of the genus Dardanus (Anomura: Diogenidae) and insights into the phylogeny of Paguroidea. Scientific Reports, 11(1): 21833. doi: 10.1038/s41598-021-01338-8 Zhang Zhan, Xing Yuhui, Cheng Jiajia, et al. 2020c. Phylogenetic implications of mitogenome rearrangements in East Asian potamiscine freshwater crabs (Brachyura: Potamidae). Molecular Phylogenetics and Evolution, 143: 106669. doi: 10.1016/j.ympev.2019.106669 -




 下载:
下载: