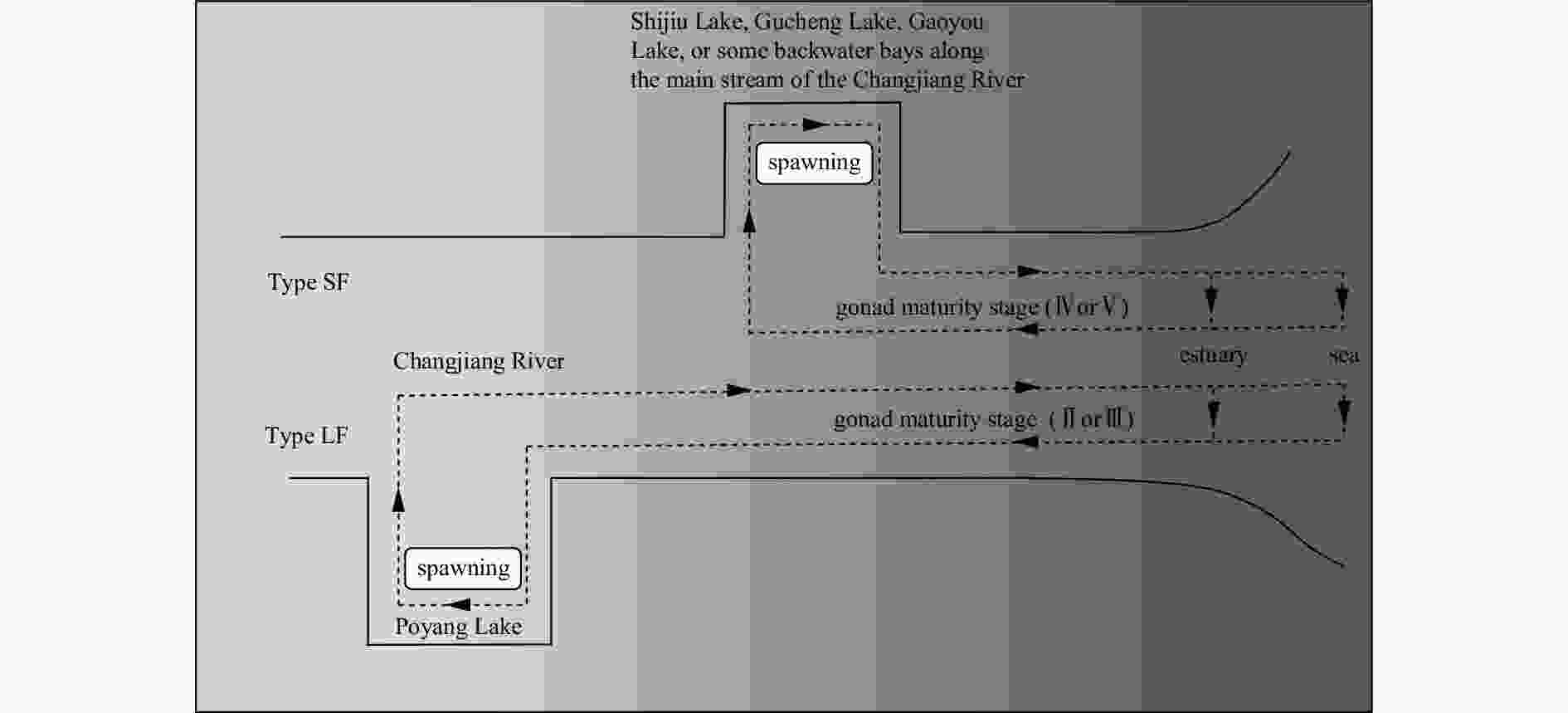
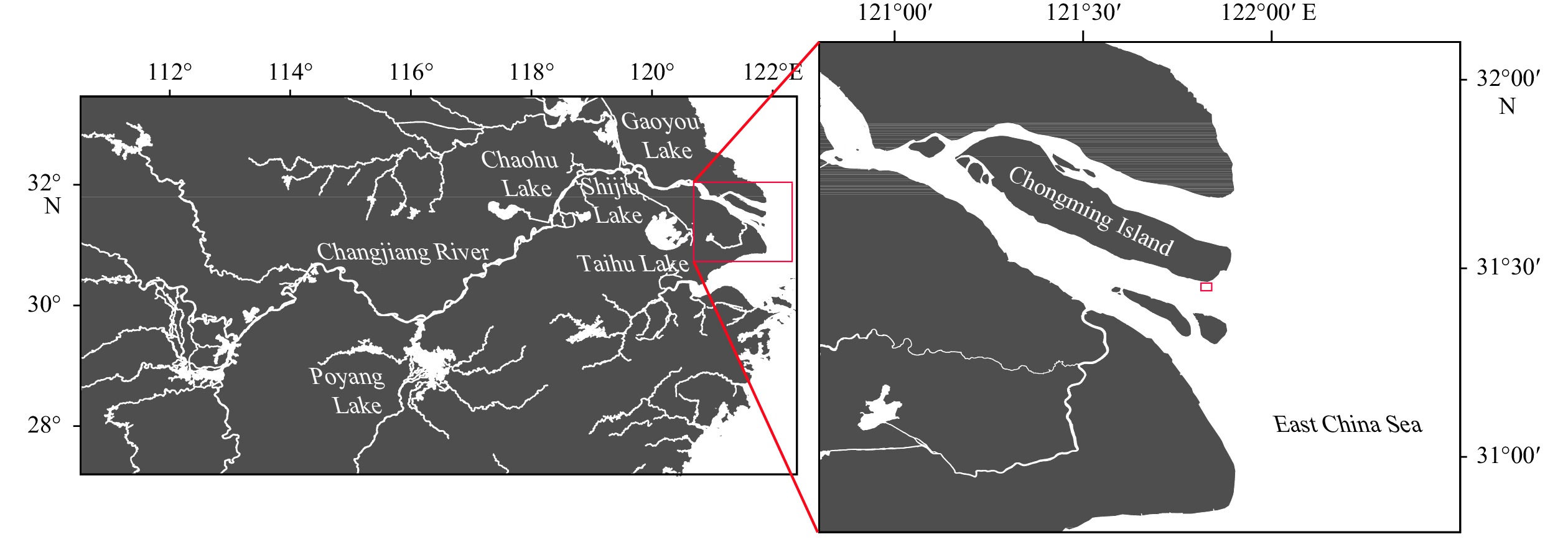
Otolith microchemical evidence revealing multiple spawning site origination of the anadromous tapertail anchovy (Coilia nasus) in the Changjiang (Yangtze) River Estuary
-
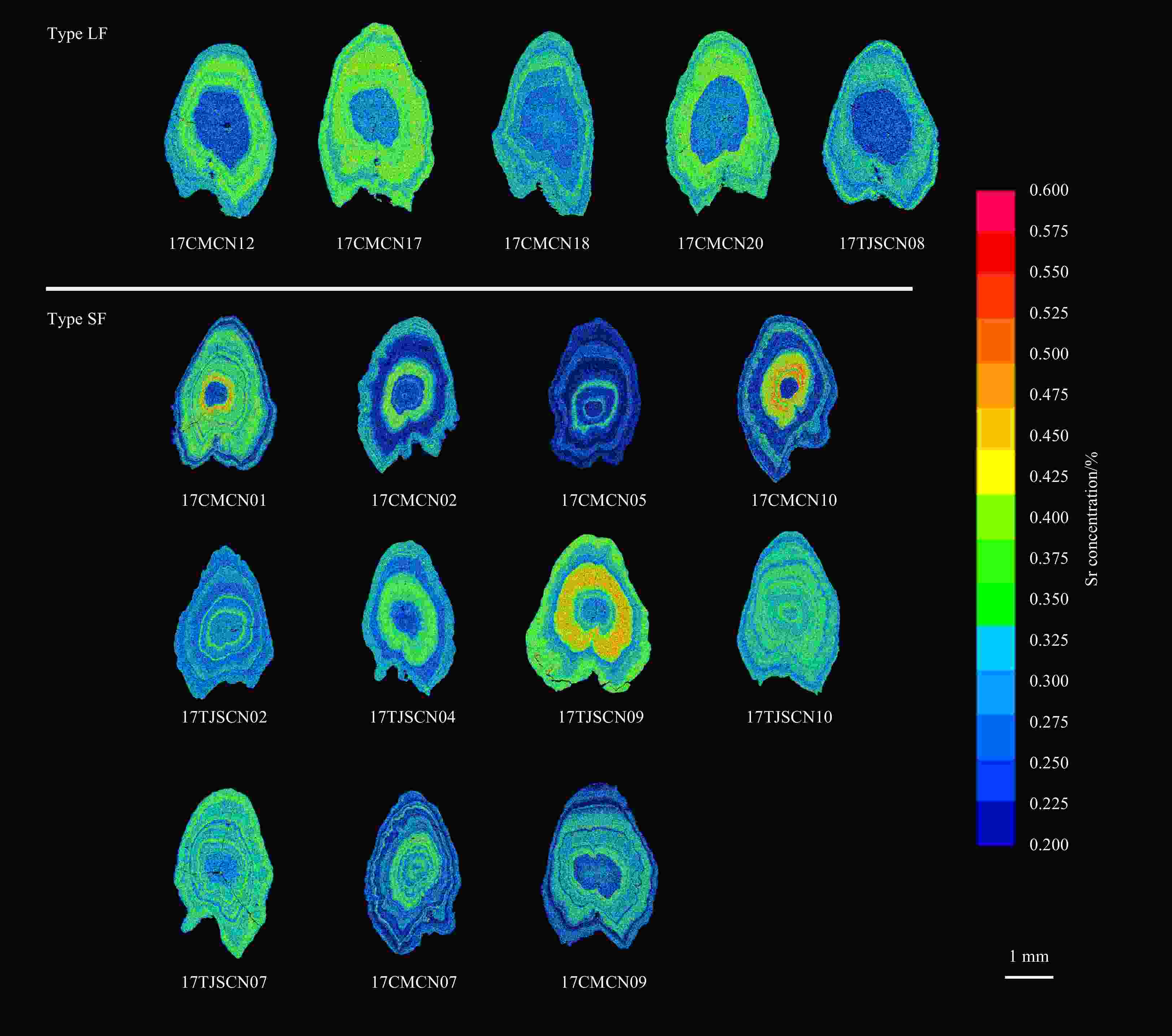
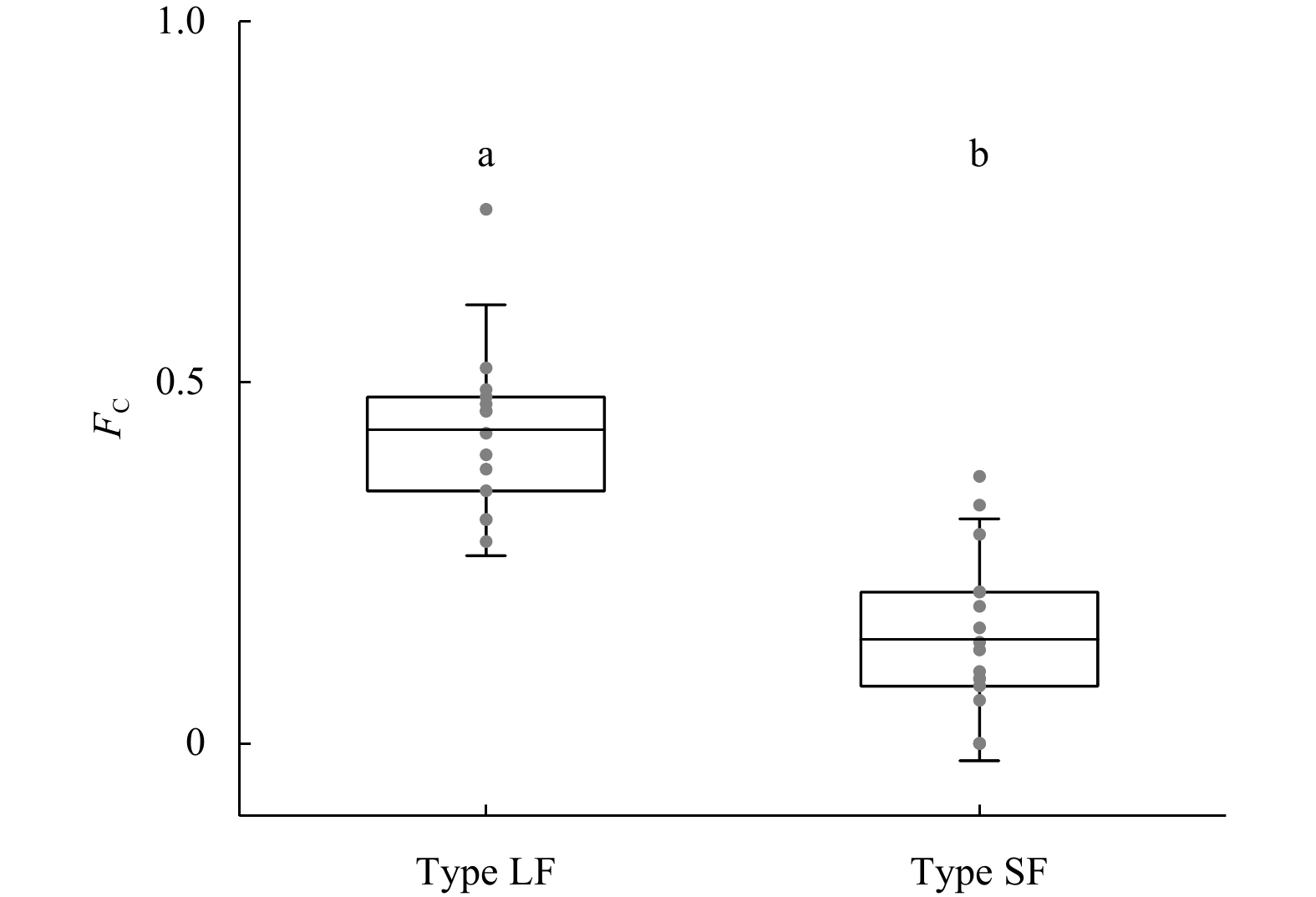
Abstract: The estuarine tapertail anchovy (Coilia nasus) is a high-value commercial fish. Estimating the spawning site or hatchery origin and habitat is essential for its conservation. This study aimed to determine the habitat use and life history characteristics of C. nasus from the Changjiang River Estuary. We investigated the environmental signatures of strontium (Sr) and calcium (Ca) in the otoliths of the collected specimens using electron probe microanalysis; additionally, we examined their gonadal maturity stage. Our results indicate that the 31 adult C. nasus specimens used in this study could be classified into two types based on their otolith Sr:Ca concentration ratios and their gonadal maturity stage. The long freshwater early life history type (Type LF) had wider central region in the otolith with low Sr:Ca concentration ratios ranging from 1.24±0.62 to 1.92±0.78 and a bluish pattern of low Sr content level. These fish are of riverine origin and had a relatively long early life history in freshwater and low gonadal maturation when captured. The short freshwater early life history type (Type SF) had a shorter central region in the otolith with low Sr:Ca concentration ratios ranging from 1.35±0.5 to 2.82±0.97 and a correspondingly bluish pattern. These fish also had a relatively short-term early life history in freshwater and high gonadal maturation when captured. The results of the otolith microchemical analysis indicated that Type LF and Type SF originated in spawning/hatching sites far from and close to the estuary, respectively. The mature gonads of Type SF fish indicated that they may breed in areas close to the estuary, whereas the immature gonads of Type LF fish indicated that they may breed in areas far from the estuary. This study is the first to reveal that the Changjiang River Estuary contains stocks of anadromous C. nasus originating in different spawning sites during the same season. The estuarine habitat plays a critical role in the connectivity between freshwater recruitment and the marine resources available to adult spawners of this commercially valued species. From a conservation perspective, this study provides important information for identifying anadromous C. nasus stocks originating in different spawning sites in the Changjiang River Basin.
-
Key words:
- Coilia nasus /
- otolith /
- migration /
- connectivity /
- spawning site
-
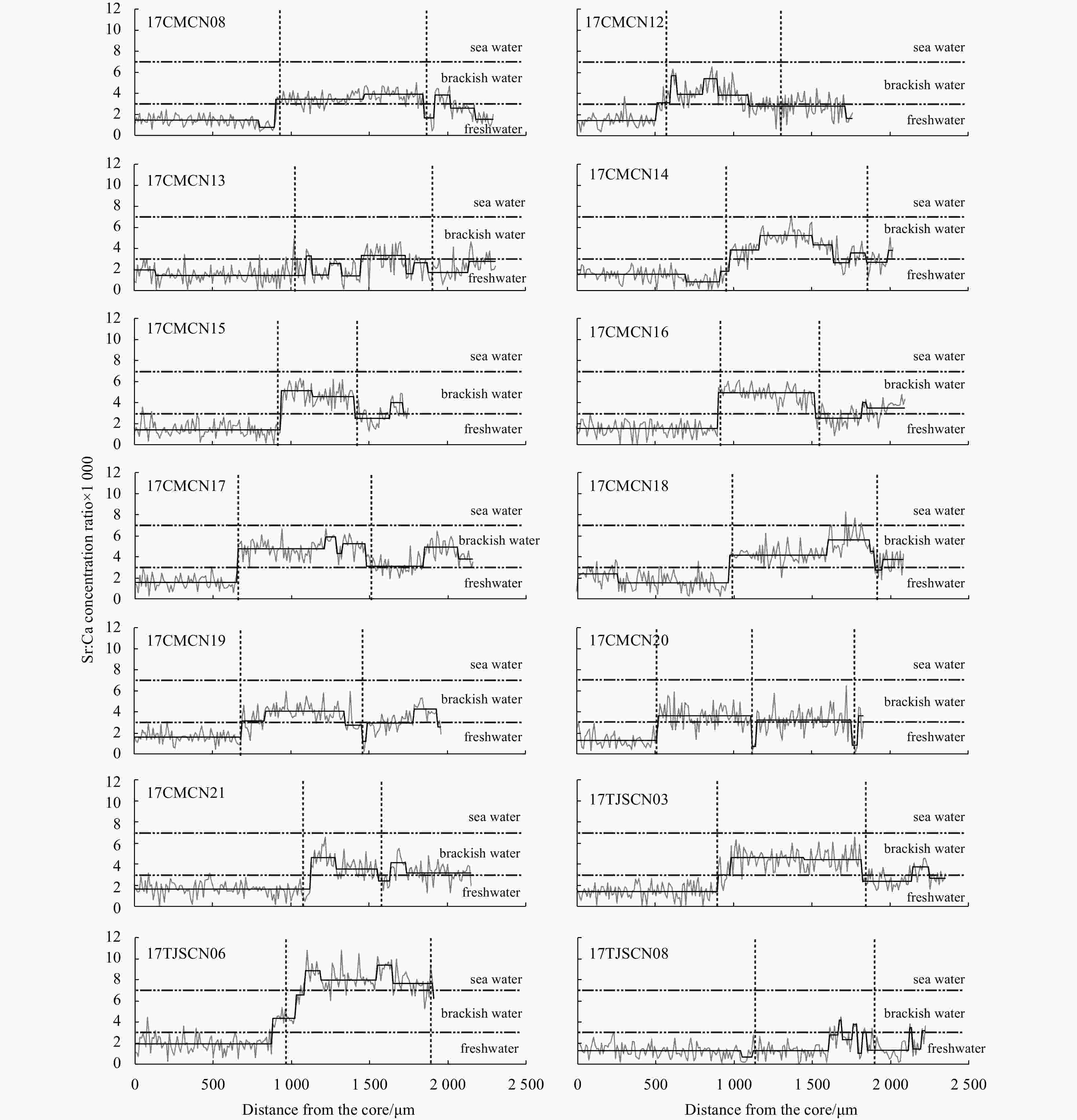
Figure 2. Fluctuation (grey line) and shift (black line) of the otolith Sr:Ca concentration ratios along line transects from the core (0 μm) to the edge of the sagittal plane of Type LF Coilia nasus, which bred far from the Changjiang River Estuary. The position of the vertical dashed lines represents the annulus.
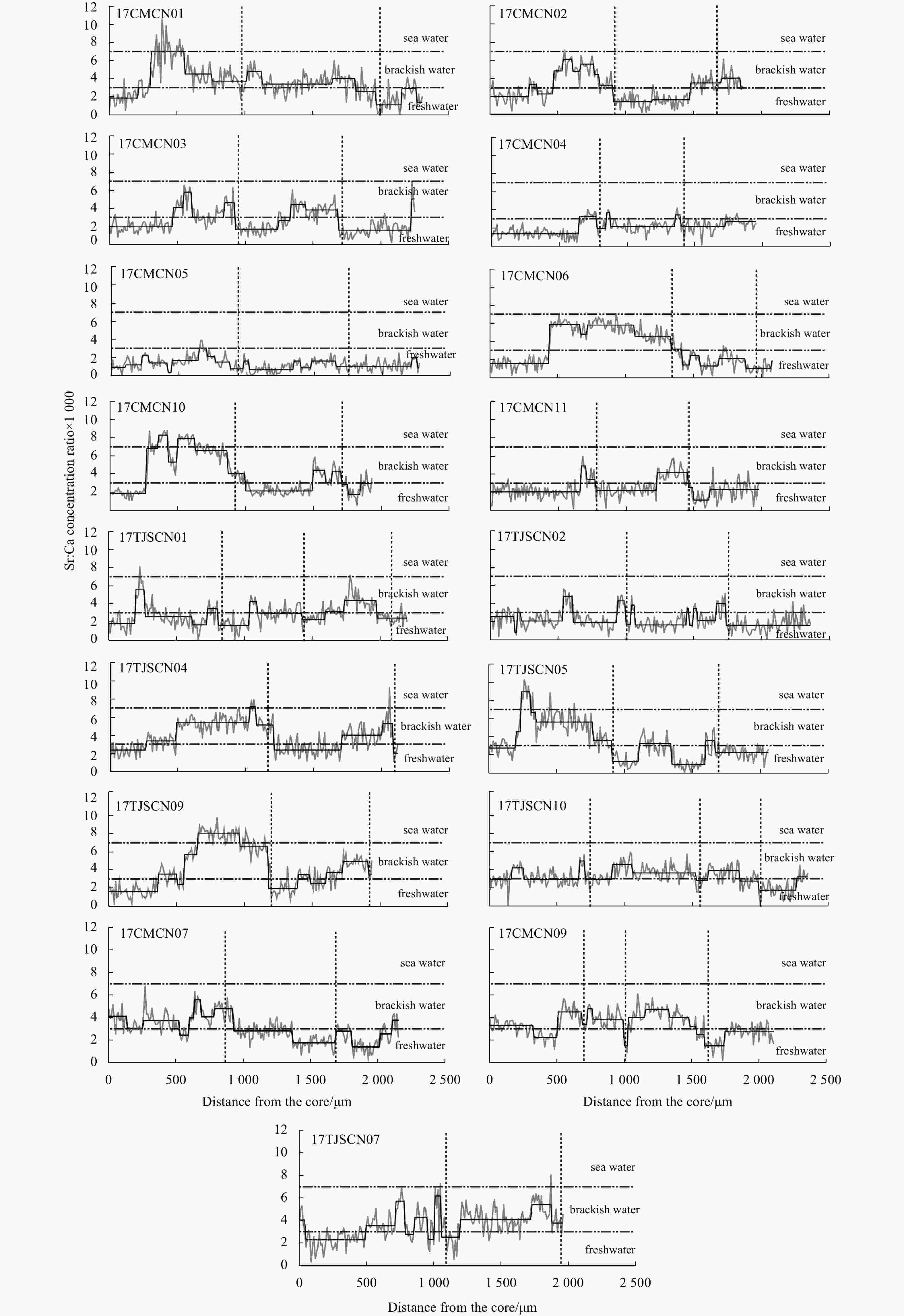
Figure 3. Fluctuation (grey line) and shift (black line) of the otolith Sr:Ca concentration ratios along the line transects from the core (0 μm) to the edge of the sagittal plane of Type SF Coilia nasus, which bred close to the Changjiang River Estuary. The position of the vertical dashed lines represents the annulus.
Figure 6. Diagrammatic model of Coilia nasus migration from the Changjiang River Estuary based on Sr:Ca concentration ratio analyses. The lines with the arrows represent possible dispersion patterns. Type LF: the long freshwater early life history type; Type SF: the short freshwater early life history type.
Table 1. The proportions of Coilia nasus specimens collected from the waters of the Changjiang River Estuary, throughout their freshwater life history
Type Individual
codeTotal length/
mmBody
weight/gSupermaxilla:Head
length ratioSex Agea Gonadal maturity
stageLfb/μm LTc/μm FCd Type LF 17CMCN08 387 140.00 1.09 ♂ 2+ II 910 2 290 0.40 17CMCN12 314 98.11 1.12 ♀ 2+ II 550 1 770 0.31 17CMCN13 309 104.01 1.24 ♀ 2+ II 1 100 2 310 0.48 17CMCN14 310 94.61 1.30 ♂ 2+ II 980 2 020 0.49 17CMCN15 317 103.62 1.26 ♂ 2+ II 980 2 100 0.47 17CMCN16 300 97.79 1.20 ♀ 2+ II 910 2 100 0.43 17CMCN17 322 115.69 1.18 ♂ 2+ II 660 2 160 0.31 17CMCN18 320 116.83 1.13 ♀ 2+ II 970 2 100 0.46 17CMCN19 294 94.36 1.21 ♂ 2+ II 690 1 960 0.35 17CMCN20 315 107.69 1.16 ♀ 3 II 510 1 830 0.28 17CMCN21 310 99.81 1.16 ♀ 2+ II 1 130 2 190 0.52 17TJSCN03 323 111.89 1.15 ♀ 2+ III 920 2 390 0.38 17TJSCN06 275 67.44 1.13 ♂ 2 III 890 1 920 0.46 17TJSCN08 333 155.13 1.22 ♂ 2+ II 1 690 2 270 0.74 Type SF 17CMCN01 325 115.81 1.24 ♀ 2+ IV 220 2 320 0.09 17CMCN02 230 31.48 1.29 ♂ 2+ V 290 1 860 0.16 17CMCN03 306 100.01 1.27 ♀ 2+ IV 480 2 250 0.21 17CMCN04 281 71.55 1.12 ♀ 2+ IV 650 1 950 0.33 17CMCN05 302 70.71 1.31 ♂ 2+ IV 660 2 270 0.29 17CMCN06 305 93.59 1.16 ♂ 2+ IV 440 2 080 0.21 17CMCN10 333 126.22 1.17 ♀ 2+ IV 270 1 930 0.14 17CMCN11 312 123.67 1.20 ♂ 2+ IV 670 1 990 0.37 17TJSCN01 322 148.16 1.14 ♀ 3+ IV 200 2 200 0.09 17TJSCN02 305 98.13 1.23 ♀ 2+ IV 200 2 370 0.08 17TJSCN04 278 72.32 1.22 ♂ 2 IV 270 2 120 0.13 17TJSCN05 295 89.19 1.35 ♀ 2+ IV 200 2 060 0.10 17TJSCN09 305 98.28 1.21 ♂ 2 IV 370 1 940 0.19 17TJSCN10 331 124.57 1.22 ♂ 3+ IV 150 2 350 0.06 17CMCN07 293 74.21 1.17 ♀ 2+ IV 0 2 160 0 17CMCN09 331 109.87 1.19 ♀ 3+ V 0 2 120 0 17TJSCN07 276 50.18 1.24 ♀ 2+ IV 0 1 970 0 Note: Type LF: the long freshwater early life history type; Type SF: the short freshwater early life history type. Agea is estimated using the otolith rings; Lf b: the length of the first freshwater stage of the low Sr:Ca concentration ratio line of the otolith microchemical line analysis or the length of the low Sr bluish central regions of the Sr mapping analysis; LTc: the radius of the entire otolith microchemical line analysis measurement line along the line down the longest axis of each otolith from the core; FCd: freshwater coefficient. -
Asaduzzaman M, Igarashi Y, Wahab M A, et al. 2020. Population genomics of an anadromous hilsa shad Tenualosa ilisha species across its diverse migratory habitats: discrimination by fine-scale local adaptation. Genes, 11(1): 46. doi: 10.3390/genes11010046 Aykanat T, Johnston S E, Orell P, et al. 2015. Low but significant genetic differentiation underlies biologically meaningful phenotypic divergence in a large Atlantic salmon population. Molecular Ecology, 24(20): 5158–5174. doi: 10.1111/mec.13383 Brown R J, Severin K P. 2009. Otolith chemistry analyses indicate that water Sr:Ca is the primary factor influencing otolith Sr:Ca for freshwater and diadromous fish but not for marine fish. Canadian Journal of Fisheries and Aquatic Sciences, 66(10): 1790–1808. doi: 10.1139/F09-112 Chen Tingting, Jiang Tao, Liu Hongbo, et al. 2017. Do all long supermaxilla-type estuarine tapertail anchovies (Coilia nasus Temminck et Schlegel, 1846) migrate anadromously?. Journal of Applied Ichthyology, 33(2): 270–273. doi: 10.1111/jai.13309 Chen Tingting, Jiang Tao, Lu Mingjie, et al. 2016. Microchemistry analysis of otoliths of Coilia nasus and Coilia brachygnathus from the Jingjiang section of the Yangtze River. Journal of Lake Sciences (in Chinese), 28(1): 149–155. doi: 10.18307/2016.0117 Cheng Fangyuan, Wang Qian, Delser P M, et al. 2019. Multiple freshwater invasions of the tapertail anchovy (Clupeiformes: Engraulidae) of the Yangtze River. Ecology and Evolution, 9(21): 12202–12215. doi: 10.1002/ece3.5708 Dai Libin, Hodgdon C, Tian Siquan, et al. 2020. Comparative performance of modelling approaches for predicting fish species richness in the Yangtze River Estuary. Regional Studies in Marine Science, 35: 101161. doi: 10.1016/j.rsma.2020.101161 Dou Shuozeng, Yokouchi K, Yu Xin, et al. 2012. The migratory history of anadromous and non-anadromous tapertail anchovy Coilia nasus in the Yangtze River Estuary revealed by the otolith Sr:Ca ratio. Environmental Biology of Fishes, 95(4): 481–490. doi: 10.1007/s10641-012-0042-1 Filina E A, Budanova L K. 2015. On the finding of mature individuals of the Greenland halibut Reinhardtius hippoglossoides (Pleuronectidae) in the Kara Sea. Journal of Ichthyology, 55(1): 138–142. doi: 10.1134/S0032945214060058 Gao Lei, Cheng Fei, Song Yiqing, et al. 2018. Patterns of larval fish assemblages along the direction of freshwater input within the southern branch of the Yangtze Estuary, China: implications for conservation. Journal of Freshwater Ecology, 33(1): 97–114. doi: 10.1080/02705060.2018.1426503 Ge Keke, Zhong Junsheng. 2010. Daily-age structure and growth characteristics of Coilia nasus larvae and juveniles in the surf zone of Yangtze River Estuary. Acta Hydrobiologica Sinica (in Chinese), 34(4): 716–721. doi: 10.3724/SP.J.1035.2010.00716 Hall C J, Jordaan A, Frisk M G. 2012. Centuries of anadromous forage fish loss: consequences for ecosystem connectivity and productivity. BioScience, 62(8): 723–731. doi: 10.1525/bio.2012.62.8.5 Hou Xuejiao, Feng Lian, Tang Jing, et al. 2020. Anthropogenic transformation of Yangtze Plain freshwater lakes: patterns, drivers and impacts. Remote Sensing of Environment, 248: 111998. doi: 10.1016/j.rse.2020.111998 Huang Liangliang, Li Jianhua. 2016. Status of freshwater fish biodiversity in the Yangtze River Basin, China. In: Nakano S I, Yahara T, Nakashizuka T, eds. Aquatic Biodiversity Conservation and Ecosystem Services. Singapore: Springer, 13–30 Huang Liangliang, Wu Zhiqiang, Li Jianhua. 2013. Fish fauna, biogeography and conservation of freshwater fish in Poyang Lake Basin, China. Environmental Biology of Fishes, 96(10): 1229–1243. doi: 10.1007/s10641-011-9806-2 Jiang Tao, Liu Hongbo, Huang Honghui, et al. 2019. Migration patterns and habitat use of the tapertail anchovy Coilia mystus in the Oujiang River Estuary and the Zhujiang River Estuary, China. Acta Oceanologica Sinica, 38(8): 35–40. doi: 10.1007/s13131-019-1436-0 Jiang Tao, Liu Hongbo, Lu Mingjie, et al. 2016. A possible connectivity among estuarine tapertail anchovy (Coilia nasus) populations in the Yangtze River, Yellow Sea, and Poyang Lake. Estuaries and Coasts, 39(6): 1762–1768. doi: 10.1007/s12237-016-0107-z Jiang Tao, Liu Hongbo, Shen Xinqiang, et al. 2014. Life history variations among different populations of Coilia nasus along the Chinese coast inferred from otolith microchemistry. Journal of the Faculty of Agriculture, Kyushu University, 59(2): 383–389, Jiang Tao, Liu Hongbo, Yang Jian. 2015a. Characteristics of C and O stable isotope in otolith of juvenile Coilia nasus from the Changjiang River Estuary. Marine Sciences (in Chinese), 39(6): 48–53. doi: 10.11759//hykx20140622001 Jiang Xiaoming, Pan Baozhu, Sun Zhiwei, et al. 2020. Application of taxonomic distinctness indices of fish assemblages for assessing effects of river-lake disconnection and eutrophication in floodplain lakes. Ecological Indicators, 110: 105955. doi: 10.1016/j.ecolind.2019.105955 Jiang Tao, Yang Jian, Liu Hongbo, et al. 2012. Life history of Coilia nasus from the Yellow Sea inferred from otolith Sr:Ca ratios. Environmental Biology of Fishes, 95(4): 503–508. doi: 10.1007/s10641-012-0066-6 Jiang Tao, Yang Jian, Lu Mingjie, et al. 2017. Discovery of a spawning area for anadromous Coilia nasus Temminck et Schlegel, 1846 in Poyang Lake, China. Journal of Applied Ichthyology, 33(2): 189–192. doi: 10.1111/jai.13293 Jiang Xuelian, Zhang Yu, Zhong Junsheng, et al. 2015b. Study on relationship between distribution of zooplankton and Coilia nasus larvae feeding features in the surf zone of Yangtze River Estuary. Resources and Environment in the Yangtze Basin (in Chinese), 24(9): 1507–1513 Karakulak S, Oray I, Corriero A, et al. 2004. Evidence of a spawning area for the bluefin tuna (Thunnus thynnus L. ) in the eastern Mediterranean. Journal of Applied Ichthyology, 20(4): 318–320. doi: 10.1111/j.1439-0426.2004.00561.x Kayaba T, Wada T, Kamiyama K, et al. 2014. Gonadal maturation and spawning migration of stocked female barfin flounder Verasper moseri off the Pacific coast of northern Japan. Fisheries Science, 80(4): 735–748. doi: 10.1007/s12562-014-0764-4 Keller D H, Zelanko P M, Gagnon J E, et al. 2018. Linking otolith microchemistry and surface water contamination from natural gas mining. Environmental Pollution, 240: 457–465. doi: 10.1016/j.envpol.2018.04.026 Khumbanyiwa D D, Li Mengmeng, Jiang Tao, et al. 2018. Unravelinghabitat use of Coilia nasus from Qiantang River of China by otolith microchemistry. Regional Studies in Marine Science, 18: 122–128. doi: 10.1016/j.rsma.2018.02.001 Li Mengmeng, Jiang Tao, Khumbanyiwa D D, et al. 2017. Reconstructing habitat history of Coilia nasus from the Hexian section of the Yangtze River in Anhui Province by otolith microchemistry. Acta Hydrobiologica Sinica (in Chinese), 41(5): 1054–1061 Li Yuxuan, Xie Songguang, Li Zhongjie, et al. 2007. Gonad development of an anadromous fish Coilia ectenes (Engraulidae) in lower reach of Yangtze River, China. Fisheries Science, 73(6): 1224–1230. doi: 10.1111/j.1444-2906.2007.01459.x Liang Cui, Pauly D. 2017. Growth and mortality of exploited fishes in China’s coastal seas and their uses for yield-per-recruit analyses. Journal of Applied Ichthyology, 33(4): 746–756. doi: 10.1111/jai.13379 Limburg K E, Waldman J R. 2009. Dramatic declines in North Atlantic diadromous fishes. BioScience, 59(11): 955–965. doi: 10.1525/bio.2009.59.11.7 Littrell K A, Ellis D, Gephard S R, et al. 2018. Evaluating the potential for prezygotic isolation and hybridization between landlocked and anadromous alewife (Alosa pseudoharengus) following secondary contact. Evolutionary Applications, 11(9): 1554–1566. doi: 10.1111/eva.12645 Liu Hongbo, Jiang Tao, Yang Jian. 2018. Unravelling habitat use of Coilia nasus from the Rokkaku River and Chikugo River estuaries of Japan by otolith strontium and calcium. Acta Oceanologica Sinica, 37(6): 52–60. doi: 10.1007/s13131-018-1190-8 Liu Fei, Lin Pengcheng, Li Mingzheng, et al. 2019. Situations and conservation strategies of fish resources in the Yangtze River Basin. Acta Hydrobiologica Sinica (in Chinese), 43: 144–156. doi: 10.7541/2019.177 Ložys L, Shiao J C, Iizuka Y, et al. 2017. Habitat use and migratory behaviour of pikeperch Sander lucioperca in Lithuanian and Latvian waters as inferred from otolith Sr:Ca ratios. Estuarine, Coastal and Shelf Science, 198: 43–52, Mattocks S, Hall C J, Jordaan A. 2017. Damming, lost connectivity, and the historical role of anadromous fish in freshwater ecosystem dynamics. BioScience, 67(8): 713–728. doi: 10.1093/biosci/bix069 McDowall R M. 2001. Diadromy, diversity and divergence: implications for speciation processes in fishes. Fish and Fisheries, 2(3): 278–285. doi: 10.1046/j.1467-2960.2001.00050.x Mei Zhigang, Cheng Peilin, Wang Kexiong, et al. 2020. A first step for the Yangtze. Science, 367(6484): 1314. doi: 10.1126/science.abb5537 Rodionov S, Overland J E. 2005. Application of a sequential regime shift detection method to the Bering Sea ecosystem. ICES Journal of Marine Science, 62(3): 328–332. doi: 10.1016/j.icesjms.2005.01.013 Santana F M, Morize E, Labonne M, et al. 2018. Connectivity between the marine coast and estuary for white mullet (Mugil curema) in northeastern Brazil revealed by otolith Sr: Ca ratio. Estuarine, Coastal and Shelf Science, 215: 124–131, Sokta L, Jiang Tao, Liu Hongbo, et al. 2020. Loss of Coilia nasus habitats in Chinese freshwater lakes: an otolith microchemistry assessment. Heliyon, 6(8): e04571. doi: 10.1016/j.heliyon.2020.e04571 Suzuki K W, Kanematsu Y, Nakayama K, et al. 2014. Microdistribution and feeding dynamics of Coilia nasus (Engraulidae) larvae and juveniles in relation to the estuarine turbidity maximum of the macrotidal Chikugo River Estuary, Ariake Sea, Japan. Fisheries Oceanography, 23(2): 157–171. doi: 10.1111/fog.12051 Taddese F, Reid M R, Closs G P. 2019. Direct relationship between water and otolith chemistry in juvenile estuarine triplefin Forsterygion nigripenne. Fisheries Research, 211: 32–39. doi: 10.1016/j.fishres.2018.11.002 Wynne M L P, Wilson K A, Limburg K E. 2015. Retrospective examination of habitat use by blueback herring (Alosa aestivalis) using otolith microchemical methods. Canadian Journal of Fisheries and Aquatic Sciences, 72(7): 1073–1086. doi: 10.1139/cjfas-2014-0206 Xu Zhi, Ma Jing, Wang Hao, et al. 2018. River discharge and saltwater intrusion level study of Yangtze River Estuary, China. Water, 10(6): 683. doi: 10.3390/w10060683 Xu Gangchun, Nie Zhijuan, Zhang Chengxiang, et al. 2012. Histological studies on testis development of Coilia nasus under artificial farming conditions. Journal of Huazhong Agricultural University (in Chinese), 31(2): 247–252 Yang Jiangshuai, Arai T, Liu Hongbo, et al. 2006. Reconstructing habitat use of Coilia mystus and Coilia ectenes of the Yangtze River Estuary, and of Coilia ectenes of Taihu Lake, based on otolith strontium and calcium. Journal of Fish Biology, 69(4): 1120–1135. doi: 10.1111/j.1095-8649.2006.01186.x Yuan Chuanmi. 1987. Spawning migration of Coilia nasus. Bulletin of Biology (in Chinese), 22(12): 1–4 Yuan Chuanmi. 1988. The resources and population composition and causes of Coilia nasus in the middle and lower reaches of the Yangtze River. Journal of Zoology (in Chinese), 23(3): 15–18 Yuan Chuanmi, Lin Jinbang, Qin Anling, et al. 1976. On the classification history and status quo of genus Coilia in China. Journal of Nanjing University: Natural Sciences Edition (in Chinese), (2): 1–12 Zhu Dongliang. 1992. Observations and studies on embryonic development and natural reproduction of Coilia nasus in Yangtze River Estuary. Fisheries Science & Technology Information (in Chinese), 19(2): 49–51 -




 下载:
下载: