Study of screening, transport pathway, and vasodilation mechanisms on angiotensin-I converting enzyme inhibitory peptide from Ulva prolifera proteins
-
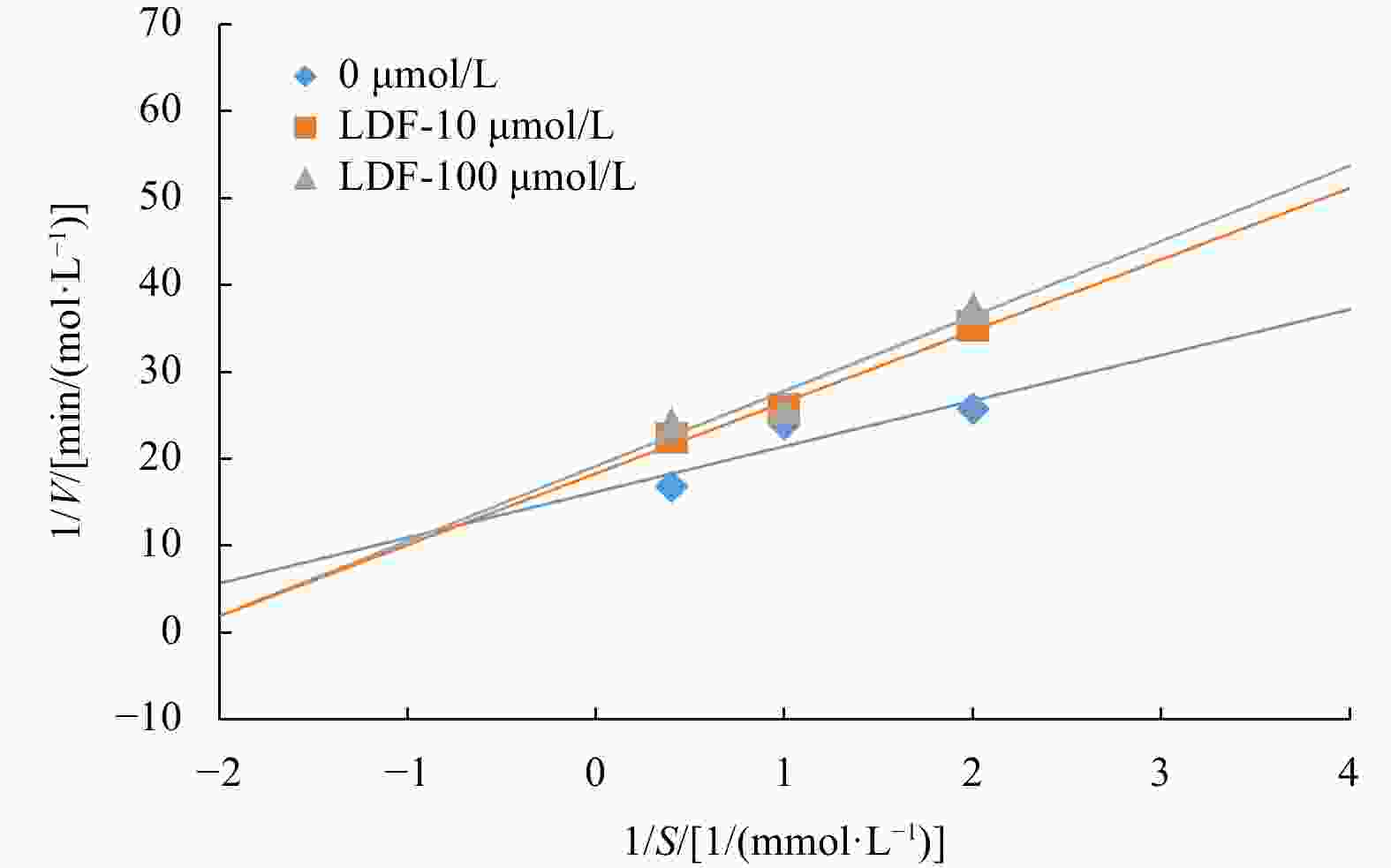
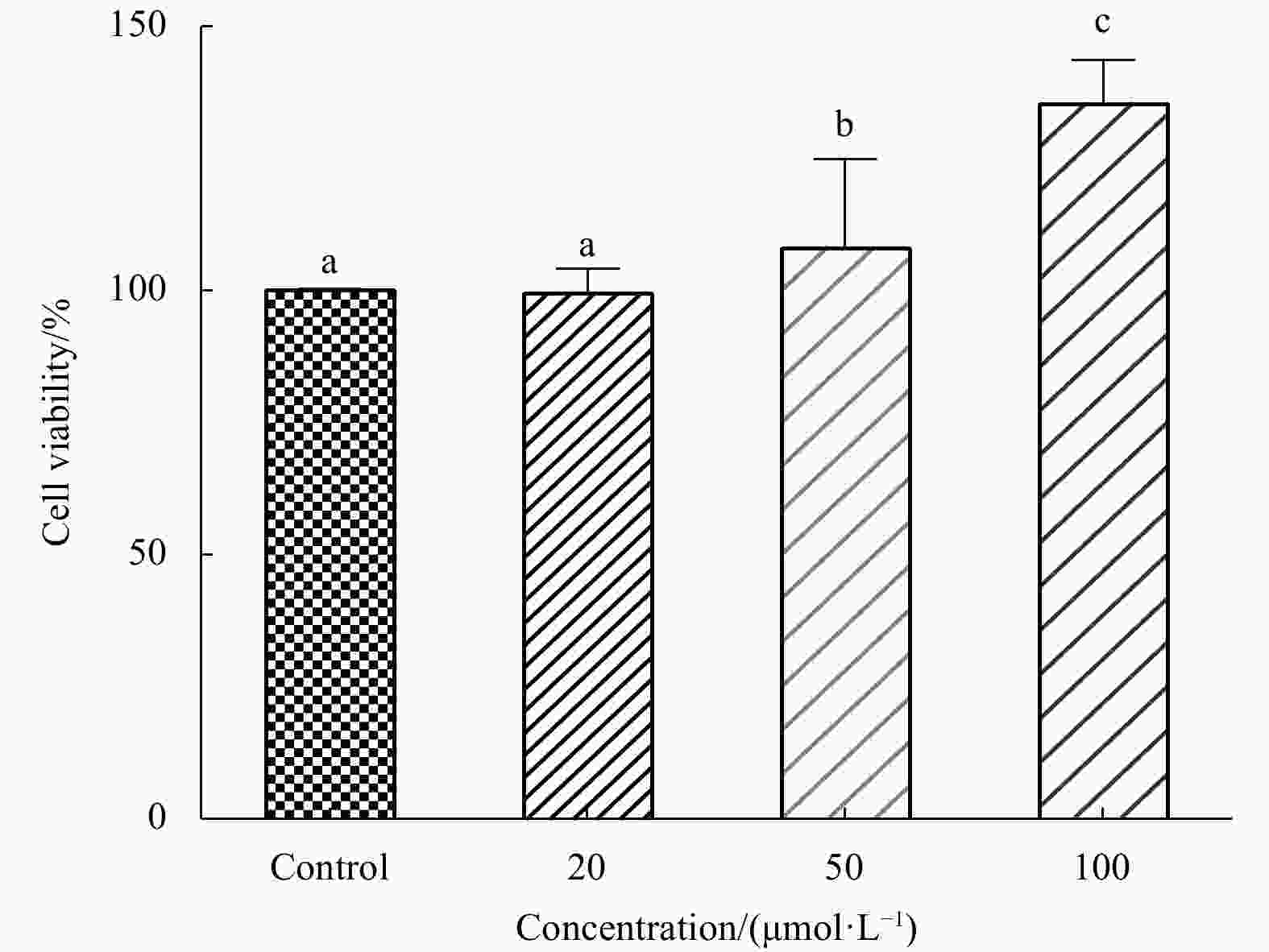
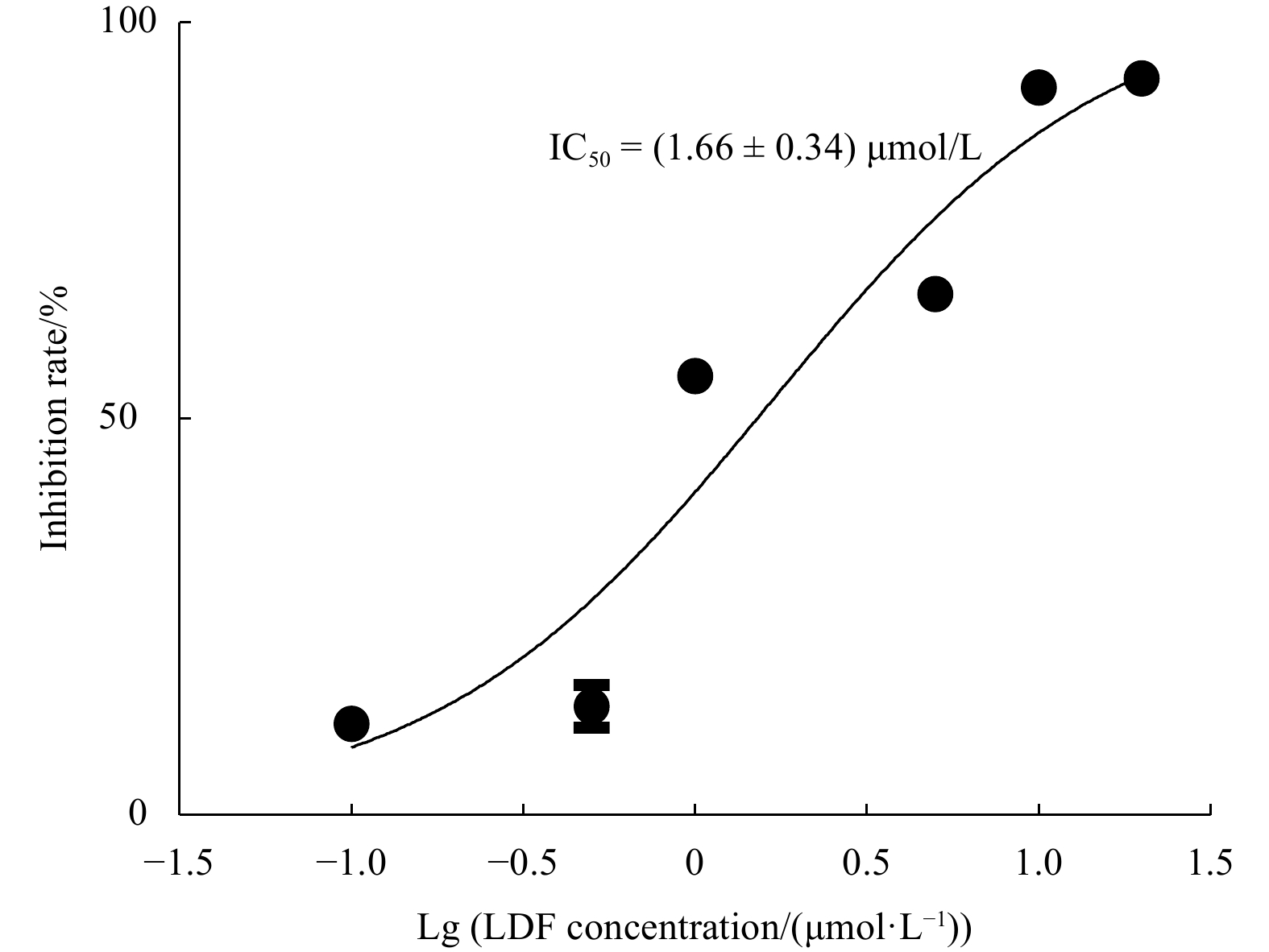
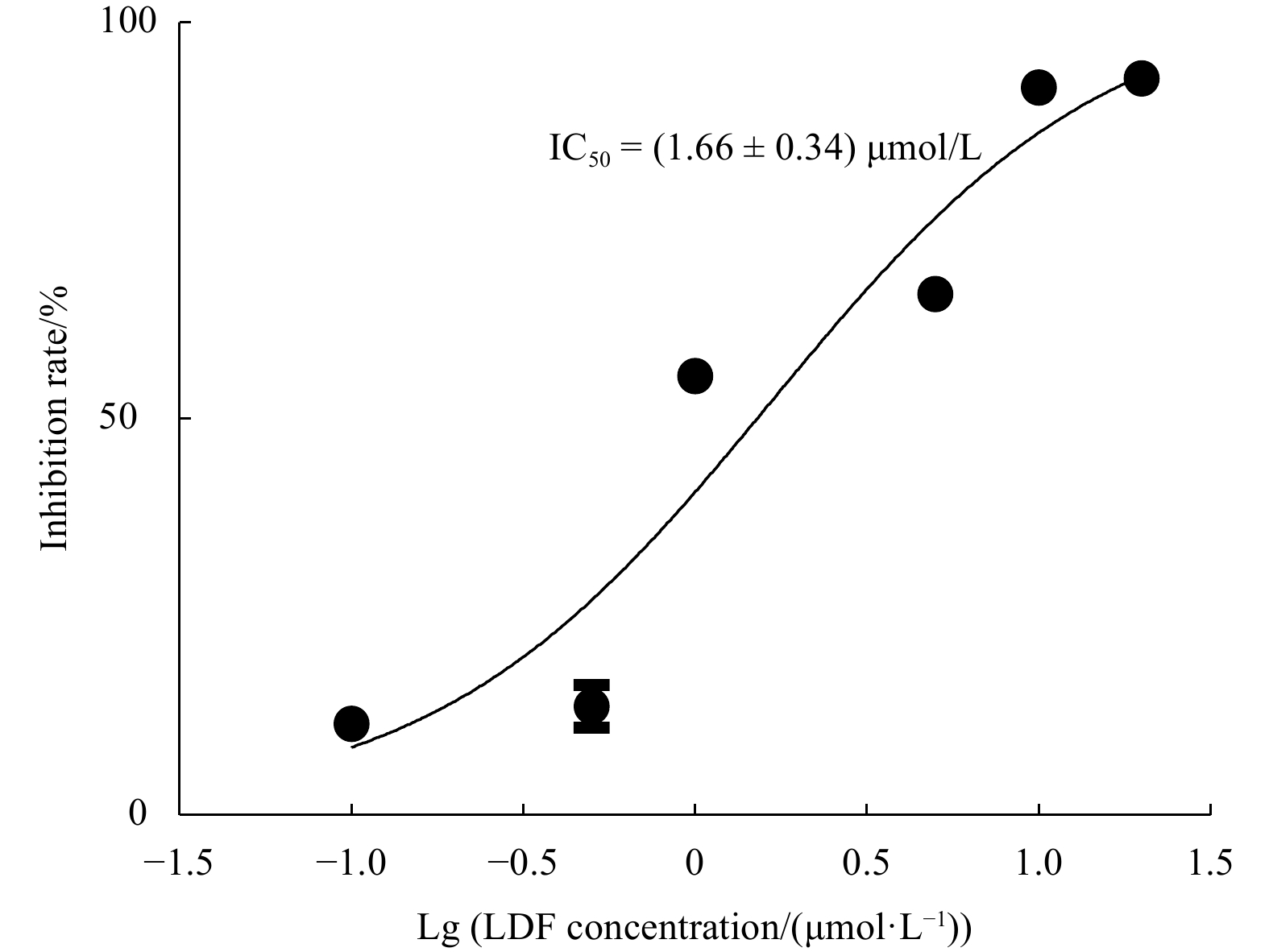
Abstract: In this study, Ulva prolifera protein was used for preparing angiotensin-I converting enzyme (ACE)-inhibitory peptide via virtual gastrointestinal digestion and in silico screening. Some parameters of the obtained peptide, such as inhibition kinetics, docking mechanism, stability, transport pathway, were explored by Lineweaver-Burk plots, molecular docking, in vitro stimulate gastrointestinal (GI) digestion and Caco-2 cells monolayer model, respectively. Then, a novel anti-ACE peptide LDF (IC50, (1.66 ± 0.34) μmol/L) was screened and synthesized by chemical synthesis. It was a no-competitive inhibitor and its anti-ACE inhibitory effect mainly attributable to four Conventional Hydrogen Bonds and Zn701 interactions. It could keep activity during simulated GI digestion in vitro and was transported by peptide transporter PepT1 and passive-mediated mode. Besides, it could activate Endothelial nitric oxide synthase (eNOS) activity to promote the production of NO and reduce Endothelin-1 (ET-1) secretion induced by Angiotensin II (Ang II) in Human Umbilical Vein Endothelial Cells (HUVECs). Meanwhile, it could promote mice splenocytes proliferation in a concentration-dependent manner. Our study indicated that this peptide was a potential ingredient functioning on vasodilation and enhancing immunity.
-
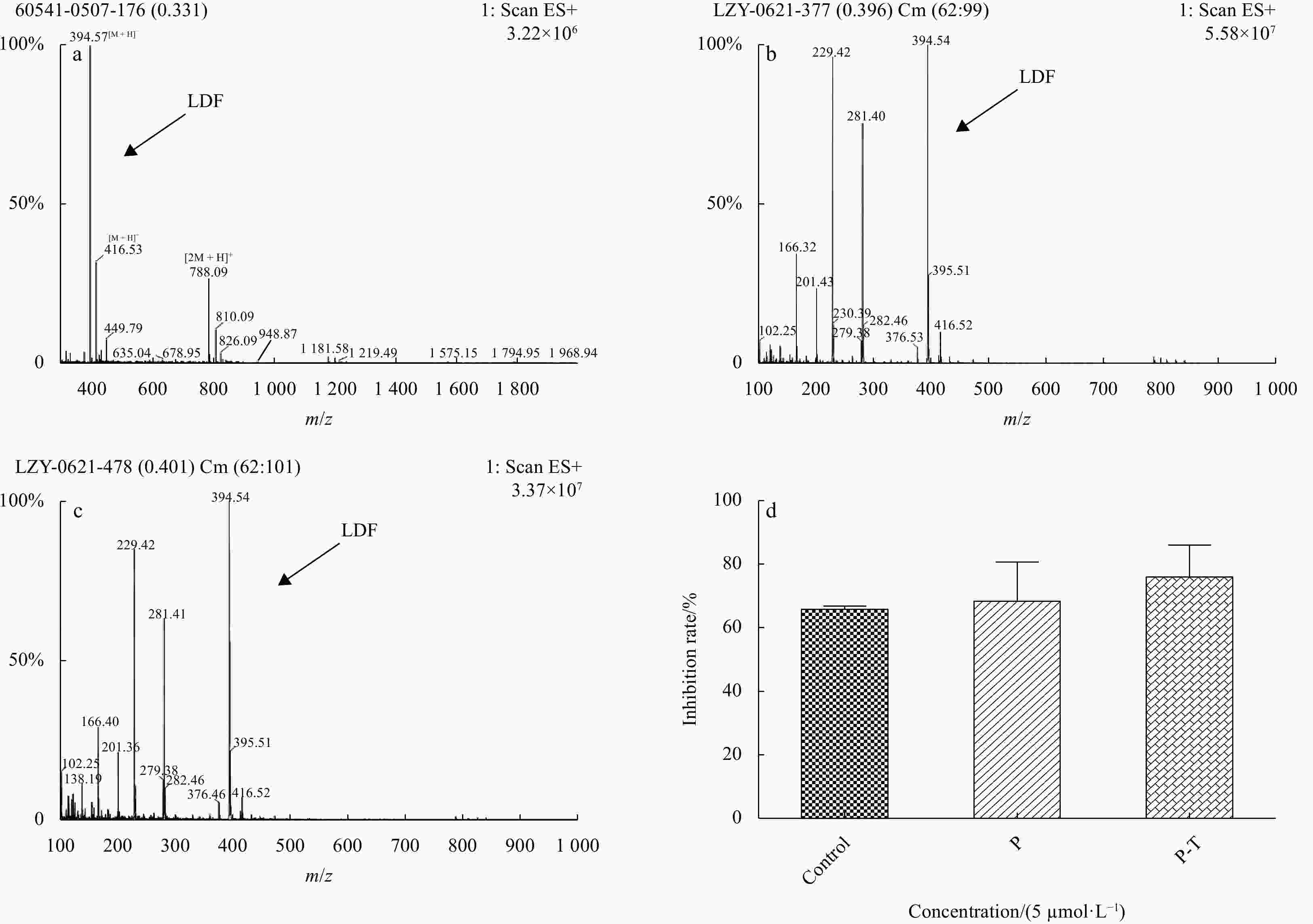
Figure 3. Stability of LDF against GI proteases. Full scan primary MS chromatogram for Control group (a), pepsin (b), pepsin-trypsin (c). d presents inhibition rate of above groups in the same concentration (P, pepsin; P-T, pepsin-trypsin; 5 μmol/L). The values are represented as the mean of the triplicate ± SD.
Figure 4. Effect of incubation time on the transport of LDF across the Caco-2 cell monolayers. a. Transport rate of LDF at different times. Samples were collected from the B side at 30 min, 60 min, and 90 min for HPLC analysis. Effect of Gly-Pro (PepT1 inhibitor), cytochalasin D (Tight junction disruptor) and wortmannin (Transcytosis inhibitor) on the transport of LDF across Caco-2 cell monolayers.Values represent the mean±standard deviation, and the bars with different lowercase letters were significantly different (p < 0.05, n = 3).
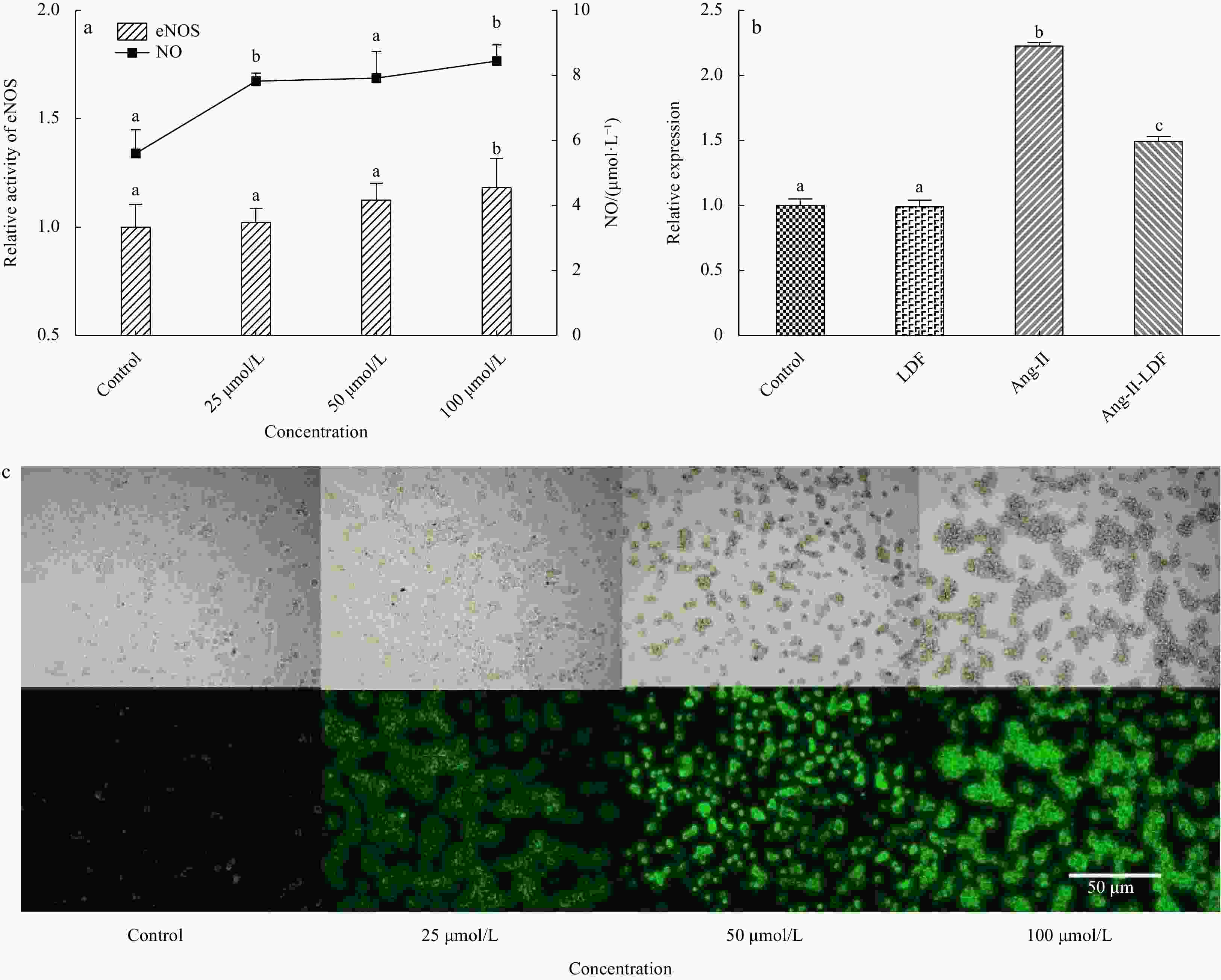
Figure 7. Effect of LDF on HUVECs. a. Effect of LDF on eNOS activity and NO secretion (extracellular). b. Effect of LDF on ET-1 secretion (Control, cells were cultured by medium for 18 h; LDF, cells were cultured by LDF for 18 h; Ang II, cells were cultured by medium for 12 h and then, adding Ang-II for another 6 h; Ang-II-LDF, cells were incubated by LDF for 12 h and then, adding Ang II for another 6 h; The final concentration of LDF and Ang II were 100 μmol/L and 100 nmol/L, respectively). c. Effect of LDF on the NO secretion (intracellular). Values (mean ± SD) that do not share a common lowercase letter within a column differ significantly (p < 0.05) (n = 3).
Table 1. Ulva prolifera protein sequences used in in silico analysis
Protein Amino acid residues Molecular weight/kDa A Pyruvate orthophosphate dikinase 899 96.43 0.1624 Adenine phosphoribosyl transferase 182 19.19 0.3908 Photosystem Ⅰ assembly protein Ycf4 185 24.42 0.4510 Ribosomal protein L14 (chloroplast) 123 13.52 0.4016 50S ribosomal protein L5 (chloroplast) 179 20.22 0.3722 30S ribosomal protein S12 (chloroplast) 74 13.60 0.3659 γ-carbonic anhydrase 1 173 23.57 0.3815 γ-carbonic anhydrase 2 226 17.99 0.3274 Plastid geranylgeranyl diphosphate synthase 330 35.69 0.2485 Plastid isopentenyl-diphosphate delta-isomerase Ⅰ 245 27.66 0.3265 Plastid 4-cytidine-5-diphospho-2-C-methyl-D-erythritol kinase 324 35.45 0.2500 Plastid 4-diphosphocytidyl-2C-methyl-D-erythritol synthase 269 29.24 0.3048 Plastid 1-deoxy-D-xylulose 5-phosphate reductoisomerase* 437 46.94 0.2517 Plastid 1-deoxy-D-xylulose 5-phosphate synthase 713 76.88 0.1865 Note: Parameter A: the frequency of bioactive fragments occurring in a protein sequence. *, represents the precursor protein for the final selected peptide. Table 2. Pool of potential ACE inhibitory peptides
Peptide Peptide ranker WS Toxin HIA BBB -CE score LDF 0.839471 Good NO +0.7591 +0.8952 75.0459 WKL 0.827822 Good NO +0.6493 +0.8556 Fail FLK 0.805333 Good NO +0.7591 +0.8371 Fail FLKF 0.956004 Good NO +0.7591 +0.8371 Fail FLPR 0.925723 Good NO +0.6554 +0.8066 Fail DLGW 0.876197 Good NO +0.6493 +0.9109 Fail LSRF 0.817020 Good NO +0.7139 +0.9595 Fail LDLF 0.806570 Good NO +0.7591 +0.8952 Fail RYIF 0.847108 Good NO +0.7519 +0.9403 Fail DFL 0.889906 Good NO +0.7591 +0.8952 Fail LDFL 0.831537 Good NO +0.7591 +0.8952 Fail LYRF 0.920545 Good NO +0.7519 +0.9186 Fail Note: WS, water solubility; HIA+, high human intestinal absorptivity; BBB +, higher blood brain barrier permeability; -CE score, score of -C Docker energy (−kcal/mol). Table 3. Interactions between ACE and candidates
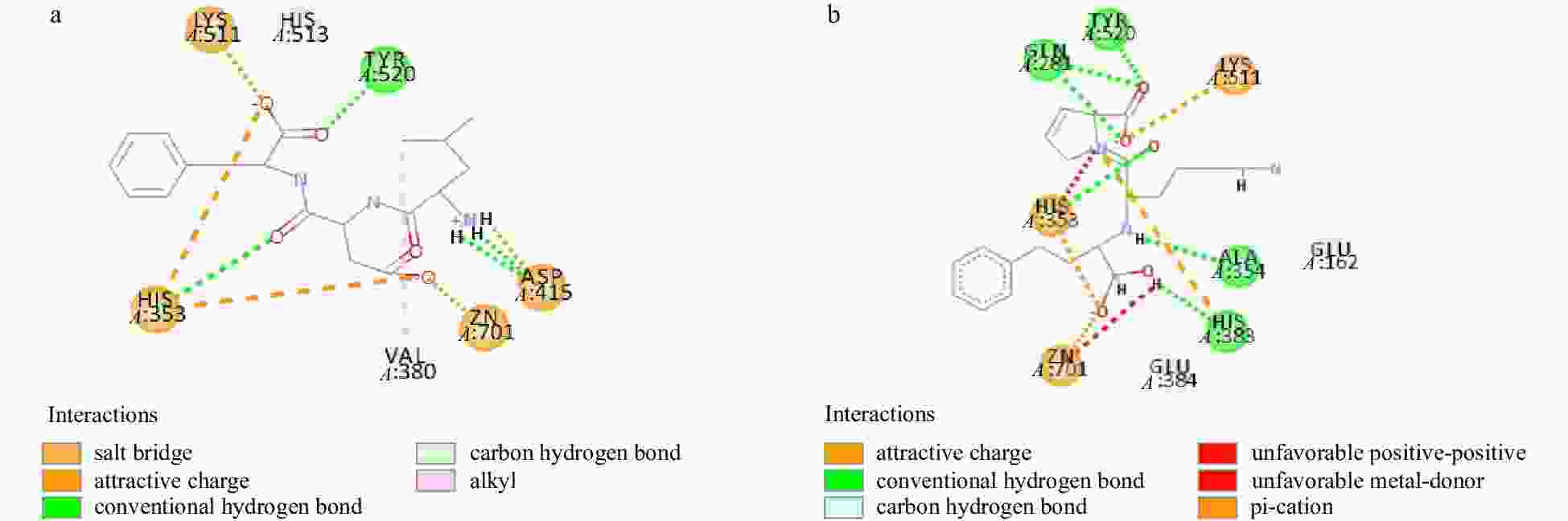
Candidates Bond position Distance/Å Type Number LDF A:LYS511:HZ3-LDF:O54 1.81686 Electrostatic interaction 5 A:ASP415:OD2-LDF:H4 2.08741 − − A:HIS353:NE2-LDF:O31 5.1492 − − A:HIS353:NE2-LDF:O54 4.02229 − − A:ZN701:ZN-LDF:O31 2.31603 − − A:HIS353:HE2-LDF:O33 2.68386 Conventional Hydrogen Bond 4 A:TYR520:HH-LDF:O53 2.287 − − A:ASP415:OD1-LDF:H2 2.52546 − − A:ASP415:OD1-LDF:H3 2.54477 − − A:HIS353:HE1-LDF:O33 2.62587 Carbon Hydrogen Bond 4 A:HIS353:HE1-LDF:O54 2.32017 − − A:VAL380:HA-LDF:O21 2.32324 − − A:HIS513:HE1-LDF:O54 2.3485 − − A:VAL380-LDF:C16 5.39525 Alkyl 1 Lisinopril (Lis) A:HIS353:NE2-Lis:O1 5.54654 Attractive Charge 3 A:LYS511:NZ-Lis:O22 5.33806 − − A:ZN701:ZN-Lis:O1 2.0673 − − A:GLN281:HE21-Lis:O22 2.45266 Conventional Hydrogen Bond 6 A:GLN281:HE22-Lis:O23 2.28218 − − A:HIS353:HE2-Lis:O17 2.46511 − − A:TYR520:HH-Lis:O23 2.02826 − − A:ALA354:O-Lis:H40 2.119 − − A:HIS383:NE2-Lis:H58 2.01119 − − A:HIS353:HE1-Lis:O17 2.54683 Carbon Hydrogen Bond 3 A:GLU162:OE2-Lis:H52 2.77882 − − A:GLU384:OE1-Lis:H59 2.29522 − − A:HIS383-Lis:N24 4.95965 Pi-Cation 1 Note: − reprents no data. -
Aguilera-Morales M, Casas-Valdez M, Carrillo-Domı́nguez S, et al. 2005. Chemical composition and microbiological assays of marine algae Enteromorpha spp. as a potential food source. Journal of Food Composition and Analysis, 18(1): 79–88. doi: 10.1016/j.jfca.2003.12.012 Balti R, Nedjar-Arroume N, Adjé E Y, et al. 2010. Analysis of novel angiotensin I-converting enzyme inhibitory peptides from enzymatic hydrolysates of cuttlefish ( Sepia officinalis) muscle proteins. Journal of Agricultural and Food Chemistry, 58(6): 3840–3846. doi: 10.1021/jf904300q Cao Dequn, Lv Xiaojing, Xu Xiaoting, et al. 2017. Purification and identification of a novel ACE inhibitory peptide from marine alga Gracilariopsis lemaneiformis protein hydrolysate. European Food Research and Technology, 243(10): 1829–1837. doi: 10.1007/s00217-017-2886-2 Chen Junbo, Yu Xiaodong, Chen Qianzi, et al. 2022. Screening and mechanisms of novel angiotensin-I-converting enzyme inhibitory peptides from rabbit meat proteins: A combined in silico and in vitro study. Food Chemistry, 370: 131070. doi: 10.1016/j.foodchem.2021.131070 Conradi R A, Wilkinson K F, Rush B D, et al. 1993. In vitro/ in vivo models for peptide oral absorption: Comparison of Caco-2 cell permeability with rat intestinal absorption of renin inhibitory peptides. Pharmaceutical Research, 10(12): 1790–1792. doi: 10.1023/A:1018990602102 Ding Xiaomeng, Hu Xiaoyi, Chen Yi, et al. 2021. Differentiated Caco-2 cell models in food-intestine interaction study: Current applications and future trends. Trends in Food Science & Technology, 107: 455–465. doi: 10.1016/j.jpgs.2020.11.015 Duerrschmidt N, Wippich N, Goettsch W, et al. 2000. Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochemical and Biophysical Research Communications, 269(3): 713–717. doi: 10.1006/bbrc.2000.2354 Ferreira L L G, Andricopulo A D. 2019. ADMET modeling approaches in drug discovery. Drug Discovery Today, 24(5): 1157–1165. doi: 10.1016/j.drudis.2019.03.015 Fukuda D, Enomoto S, Nagai R, et al. 2009. Inhibition of renin–angiotensin system attenuates periadventitial inflammation and reduces atherosclerotic lesion formation. Biomedicine & Pharmacotherapy, 63(10): 754–761. doi: 10.1016/j.biopha.2009.02.006 Furuta T, Miyabe Y, Yasui H, et al. 2016. Angiotensin I converting enzyme inhibitory peptides derived from phycobiliproteins of dulse Palmaria palmata. Marine Drugs, 14(2): 32. doi: 10.3390/md14020032 García-Tejedor A, Gimeno-Alcañíz J V, Tavárez S, et al. 2015. An antihypertensive lactoferrin hydrolysate inhibits angiotensin I-converting enzyme, modifies expression of hypertension-related genes and enhances nitric oxide production in cultured human endothelial cells. Journal of Functional Foods, 12: 45–54. doi: 10.1016/j.jff.2014.11.002 Guo Huimin, Richel A, Hao Yuqiong, et al. 2020. Novel dipeptidyl peptidase-IV and angiotensin-I-converting enzyme inhibitory peptides released from quinoa protein by in silico proteolysis. Food Science & Nutrition, 8(3): 1415–1422. doi: 10.1002/fsn3.1423 He Yuan, Shen Songdong, Yu Dachun, et al. 2021. The Ulva prolifera genome reveals the mechanism of green tides. Journal of Oceanology and Limnology, 39(4): 1458–1470. doi: 10.1007/s00343-020-0212-5 Hidalgo I J, Raub T J, Borchardt R T. 1989. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology, 96(3): 736–749. doi: 10.1016/0016-5085(89)90897-4 Horiguchi N, Horiguchi H, Suzuki Y. 2005. Effect of wheat gluten hydrolysate on the immune system in healthy human subjects. Bioscience, Biotechnology, and Biochemistry, 69(12): 2445–2449. Hou Hu, Fan Yan, Li Bafang, et al. 2012. Purification and identification of immunomodulating peptides from enzymatic hydrolysates of Alaska pollock frame. Food Chemistry, 134(2): 821–828. doi: 10.1016/j.foodchem.2012.02.186 Imai T, Hirata Y, Emori T, et al. 1992. Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension, 19(6_pt_2): 753–757. doi: 10.1161/01.HYP.19.6.753 Iwaniak A, Minkiewicz P, Pliszka M, et al. 2020. Characteristics of biopeptides released in silico from collagens using quantitative parameters. Foods, 9(7): 965. doi: 10.3390/foods9070965 Ko S C, Kang N, Kim E A, et al. 2012. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochemistry, 47(12): 2005–2011. doi: 10.1016/j.procbio.2012.07.015 Kumagai Y, Kitade Y, Kobayashi M, et al. 2020. Identification of ACE inhibitory peptides from red alga Mazzaella japonica. European Food Research and Technology, 246(11): 2225–2231. doi: 10.1007/s00217-020-03567-z Lacroix I M E, Chen Xiumin, Kitts D D, et al. 2017. Investigation into the bioavailability of milk protein-derived peptides with dipeptidyl-peptidase IV inhibitory activity using Caco-2 cell monolayers. Food & Function, 8(2): 701–709. doi: 10.1039/C6FO01411A Lamping K, Faraci F. 2003. Enhanced vasoconstrictor responses in eNOS deficient mice. Nitric Oxide, 8(4): 207–213. doi: 10.1016/S1089-8603(03)00028-4 Li Hongmei, Zhang Yongyu, Han Xiurong, et al. 2016. Growth responses of Ulva prolifera to inorganic and organic nutrients: Implications for macroalgal blooms in the southern Yellow Sea, China. Scientific Reports, 6(1): 26498. doi: 10.1038/srep26498 Li Zhiyong, Zhao Shan, Xin Xiangdong, et al. 2020. Purification, identification and functional analysis of a novel immunomodulatory peptide from silkworm pupa protein. International Journal of Peptide Research and Therapeutics, 26(1): 243–249. doi: 10.1007/s10989-019-09832-4 Lin Kai, Ma Zhao, Ramachandran M, et al. 2020. ACE inhibitory peptide KYIPIQ derived from yak milk casein induces nitric oxide production in HUVECs and diffuses via a transcellular mechanism in Caco-2 monolayers. Process Biochemistry, 99: 103–111. doi: 10.1016/j.procbio.2020.08.031 Lin Kai, Zhang Lanwei, Han Xue, et al. 2017. Novel angiotensin I-converting enzyme inhibitory peptides from protease hydrolysates of Qula casein: Quantitative structure-activity relationship modeling and molecular docking study. Journal of Functional Foods, 32: 266–277. doi: 10.1016/j.jff.2017.03.008 Lin Kai, Zhang Lanwei, Han Xue, et al. 2018. Yak milk casein as potential precursor of angiotensin I-converting enzyme inhibitory peptides based on in silico proteolysis. Food Chemistry, 254: 340–347. doi: 10.1016/j.foodchem.2018.02.051 Liu Ping, Liao Wang, Qi Xingpu, et al. 2020. Identification of immunomodulatory peptides from zein hydrolysates. European Food Research and Technology, 246(5): 931–937. doi: 10.1007/s00217-020-03450-x Liu Yunmeng, Rafferty T M, Rhee S W, et al. 2017. CD8+ T cells stimulate Na-Cl co-transporter NCC in distal convoluted tubules leading to salt-sensitive hypertension. Nature Communications, 8(1): 14037. doi: 10.1038/ncomms14037 Maeno M, Yamamoto N, Takano T. 1996. Identification of an antihypertensive peptide from casein hydrolysate produced by a proteinase from Lactobacillus helveticus CP790. Journal of Dairy Science, 79(8): 1316–1321. doi: 10.3168/jds.S0022-0302(96)76487-1 Majumder K, Wu Jianping. 2009. Angiotensin I converting enzyme inhibitory peptides from simulated in vitro gastrointestinal digestion of cooked eggs. Journal of Agricultural and Food Chemistry, 57(2): 471–477. doi: 10.1021/jf8028557 Mao Ruixue, Wu Lan, Zhu Na, et al. 2020. Immunomodulatory effects of walnut ( Juglans regia L. ) oligopeptides on innate and adaptive immune responses in mice. Journal of Functional Foods, 73: 104068. doi: 10.1016/j.jff.2020.104068 Mills K T, Stefanescu A, He Jiang. 2020. The global epidemiology of hypertension. Nature Reviews Nephrology, 16(4): 223–237. doi: 10.1038/s41581-019-0244-2 Miyauchi T, Tomobe Y, Shiba R, et al. 1990. Involvement of endothelin in the regulation of human vascular tonus. Potent vasoconstrictor effect and existence in endothelial cells. Circulation, 81(6): 1874–1880. doi: 10.1161/01.CIR.81.6.1874 Natesh R, Schwager S L U, Sturrock E D, et al. 2003. Crystal structure of the human angiotensin-converting enzyme–lisinopril complex. Nature, 421(6922): 551–554. doi: 10.1038/nature01370 Saikun Pan, Shujun Wang , Lingling Jing , Dongrui Yao, et al. 2016. Purification and characterisation of a novel angiotensin-I converting enzyme (ACE)-inhibitory peptide derived from the enzymatic hydrolysate of Enteromorpha clathrata protein. Food Chemistry, 211: 423–430. doi: 10.1016/j.foodchem.2016.05.087 Pei Jingyan, Hua Ying, Zhou Tingyi, et al. 2021. Transport, in vivo antihypertensive effect, and pharmacokinetics of an Angiotensin-Converting Enzyme (ACE) inhibitory peptide LVLPGE. Journal of Agricultural and Food Chemistry, 69(7): 2149–2156. doi: 10.1021/acs.jafc.0c07048 Raghavan S, Kristinsson H G. 2009. ACE-inhibitory activity of tilapia protein hydrolysates. Food Chemistry, 117(4): 582–588. doi: 10.1016/j.foodchem.2009.04.058 Robb G B, Carson A R, Tai S C, et al. 2004. Post-transcriptional regulation of endothelial nitric-oxide synthase by an overlapping antisense mRNA transcript. Journal of Biological Chemistry, 279(36): 37982–37996. doi: 10.1074/jbc.M400271200 Sütas Y, Soppi E, Korhonen H, et al. 1996. Suppression of lymphocyte proliferation in vitro by bovine caseins hydrolyzed with Lactobacillus casei GG–derived enzymes. Journal of Allergy and Clinical Immunology, 98(1): 216–224. doi: 10.1016/S0091-6749(96)70245-2 Sangsawad P, Choowongkomon K, Kitts D D, et al. 2018. Transepithelial transport and structural changes of chicken angiotensin I-converting enzyme (ACE) inhibitory peptides through Caco-2 cell monolayers. Journal of Functional Foods, 45: 401–408. doi: 10.1016/j.jff.2018.04.020 Sessa W C. 2004. eNOS at a glance. Journal of Cell Science, 117(12): 2427–2429. doi: 10.1242/jcs.01165 Sumikawa K, Takei K, Kumagai Y, et al. 2020. In silico analysis of ACE inhibitory peptides from chloroplast proteins of red alga Grateloupia asiatica. Marine Biotechnology, 22(3): 391–402. doi: 10.1007/s10126-020-09959-2 Tan I H, Blomster J, Hansen G, et al. 1999. Molecular phylogenetic evidence for a reversible morphogenetic switch controlling the gross morphology of two common genera of green seaweeds, Ulva and Enteromorpha. Molecular Biology and Evolution, 16(8): 1011–1018. doi: 10.1093/oxfordjournals.molbev.a026190 Udenigwe C C. 2014. Bioinformatics approaches, prospects and challenges of food bioactive peptide research. Trends in Food Science & Technology, 36(2): 137–143. doi: 10.1016/j.jpgs.2014.02.004 Udenigwe C C. 2016. Towards rice bran protein utilization: In silico insight on the role of oryzacystatins in biologically-active peptide production. Food Chemistry, 191: 135–138. doi: 10.1016/j.foodchem.2015.01.043 Wen Li, Huang Lu, Li Yiwei, et al. 2021. New peptides with immunomodulatory activity identified from rice proteins through peptidomic and in silico analysis. Food Chemistry, 364: 130357. doi: 10.1016/j.foodchem.2021.130357 Xie Jingli, Chen Xujun, Wu Junjie, et al. 2018. Antihypertensive effects, molecular docking study, and isothermal titration calorimetry assay of angiotensin I-converting enzyme inhibitory peptides from Chlorella vulgaris. Journal of Agricultural and Food Chemistry, 66(6): 1359–1368. doi: 10.1021/acs.jafc.7b04294 Xu Qingbiao, Fan Hongbing, Yu Wenlin, et al. 2017. Transport study of egg-derived antihypertensive peptides (LKP and IQW) using Caco-2 and HT29 coculture monolayers. Journal of Agricultural and Food Chemistry, 65(34): 7406–7414. doi: 10.1021/acs.jafc.7b02176 Xu Zhenqiu, Wu Changping, Sun-Waterhouse D, et al. 2021. Identification of post-digestion angiotensin-I converting enzyme (ACE) inhibitory peptides from soybean protein isolate: Their production conditions and in silico molecular docking with ACE. Food Chemistry, 345: 128855. doi: 10.1016/j.foodchem.2020.128855 Yang Qian, Cai Xixi, Huang Muchen, et al. 2020. A specific peptide with immunomodulatory activity from Pseudostellaria heterophylla and the action mechanism. Journal of Functional Foods, 68: 103887. doi: 10.1016/j.jff.2020.103887 Yang Ruiyue, Zhang Zhaofeng, Pei Xinrong, et al. 2009. Immunomodulatory effects of marine oligopeptide preparation from Chum Salmon ( Oncorhynchus keta) in mice. Food Chemistry, 113(2): 464–470. doi: 10.1016/j.foodchem.2008.07.086 Ye Naihao, Zhang Xiaowen, Mao Yuze, et al. 2011. ‘Green tides’ are overwhelming the coastline of our blue planet: taking the world’s largest example. Ecological Research, 26(3): 477–485. doi: 10.1007/s11284-011-0821-8 Zabel U, Weeger M, La M, et al. 1998. Human soluble guanylate cyclase: functional expression and revised isoenzyme family. Biochemical Journal, 335(1): 51–57. doi: 10.1042/bj3350051 -




 下载:
下载: