Photosynthetic physiology and stress-resistant biochemical properties reveal the invasive photo-adaptation strategy of marine green alga Codium fragile
-
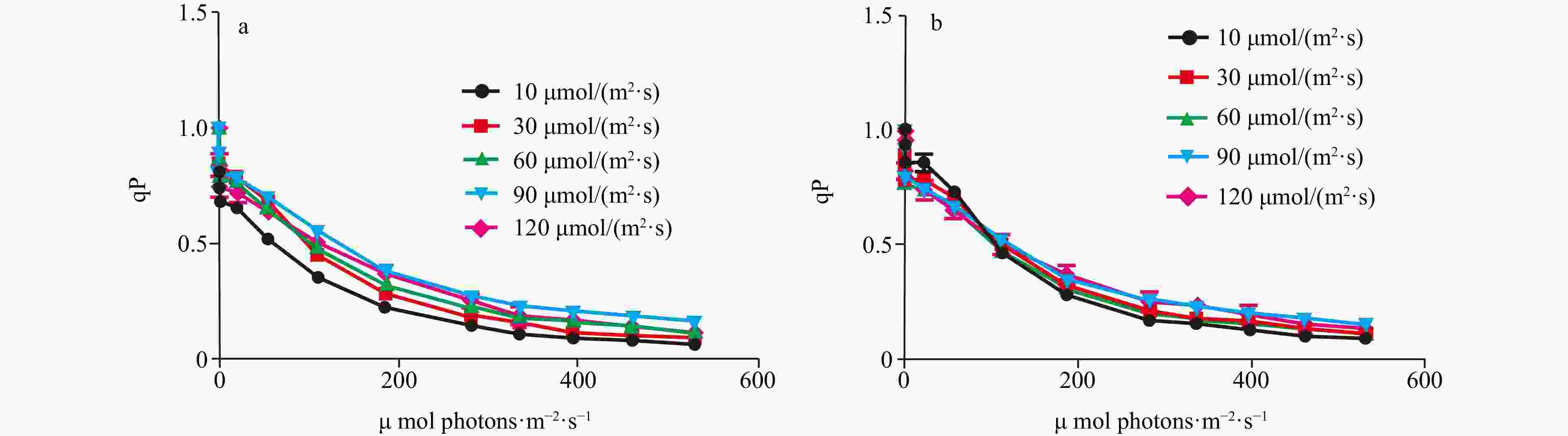
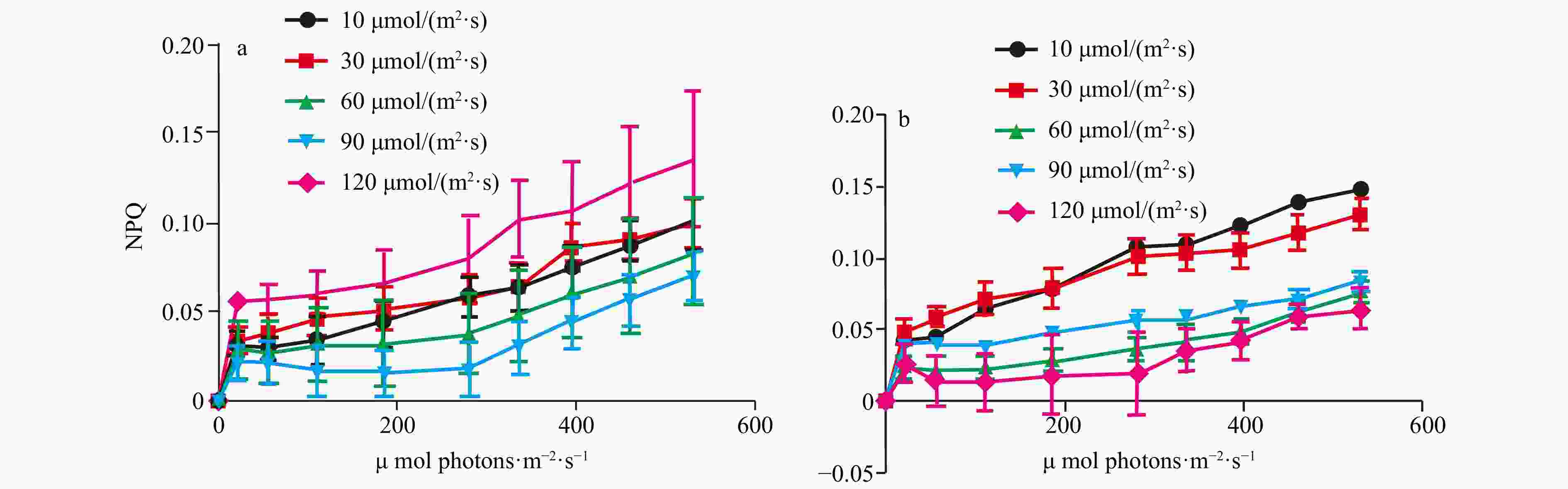
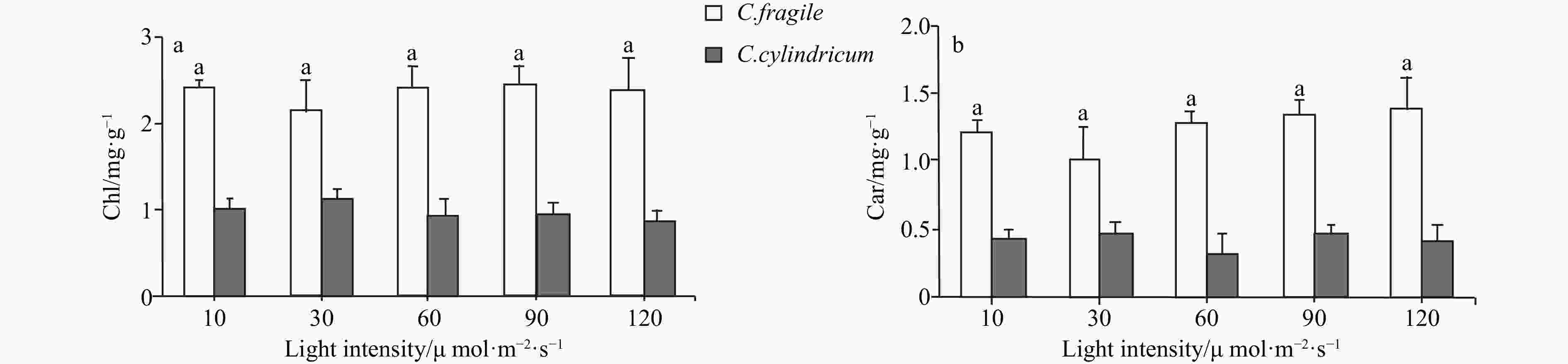
Abstract: Biological invasions have become recognized as one of the greatest threats to ecosystems. Codium, a genus of invasive green algae, has frequent global outbreaks and damages local marine ecosystems. It is now generally accepted that light is one of the main factors affecting the luxuriant growth of macroalgae such as Codium. In this study, to investigate the invasive photo-adaptation strategy of Codium fragile, the photo-adaptation characteristics of C. fragile and C. cylindricum from the Nan’ ao Island of China were compared and explored. The effect of light intensity on the photosynthetic properties of the two species was investigated: the maximum quantum yield of photosystem II (Fv/Fm) of C. fragile was significantly higher at low light intensity. At a light intensity of 90 μmol/(m2·s), maximum relative electron transport rate (rETRmax) of the thalli was maximum, and the minimum saturating irradiance (Ek) was significantly increased. The photosynthetic rate (α value) of thalli was highest at a light intensity of 30 μmol/(m2·s). The photochemical quenching (qP) was enhanced but non-photochemical quenching (NPQ) was reduced at high light intensities. As for C. cylindricum, the optimal photochemical efficiency of the thalli at low light intensity was higher. High light intensity significantly reduced the rETR of the thalli. At low light intensity, α was significantly higher, Ek was significantly lower, and NPQ was also significantly decreased. The response relationship between light acclimation and antioxidant capacity of the thalli of two species of Codium was investigated: there was no significant effect of light intensity variation on the total antioxidant capacity of C. fragile. In the case of C. cylindricum, the degree of membrane lipid peroxidation was significantly increased at low light intensity, and its antioxidant capacity was significantly reduced when the light intensity was too high or too low. It can be hypothesized that the self-protection ability of C. fragile may be stronger than that of C. cylindricum under low and high light intensities, which is closely related to the strong invasiveness of C. fragile.
-
Key words:
- Codium fragile /
- invasive algae /
- photo-adaptation /
- photosynthesis
-
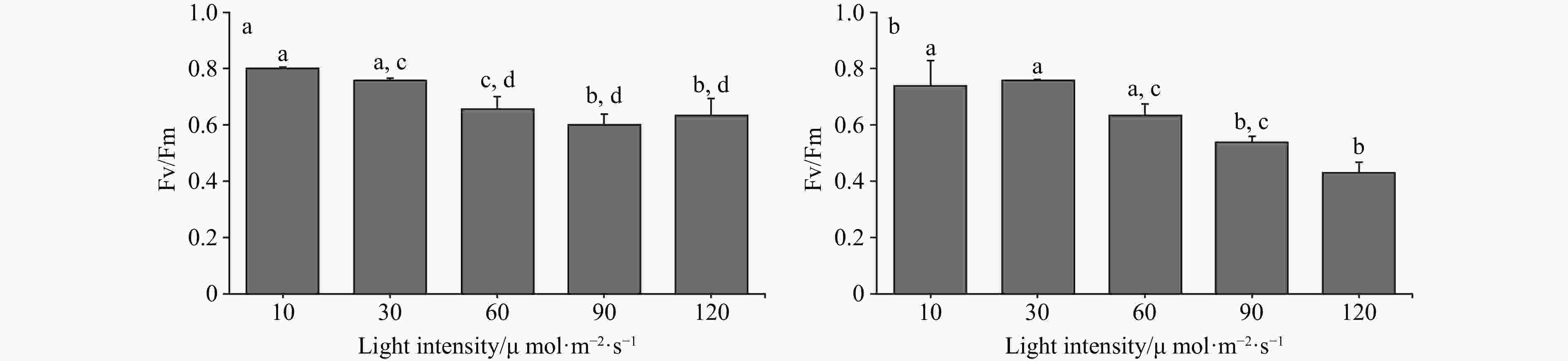
Figure 1. The Fv/Fm of two Codium species cultured at different light intensities. a. The Fv/Fm of C. fragile cultured at different light intensities, and b. the Fv/Fm of C. cylindricum cultured at different light intensities. Different letters (a, b, c, and d) represent significant differences among groups.
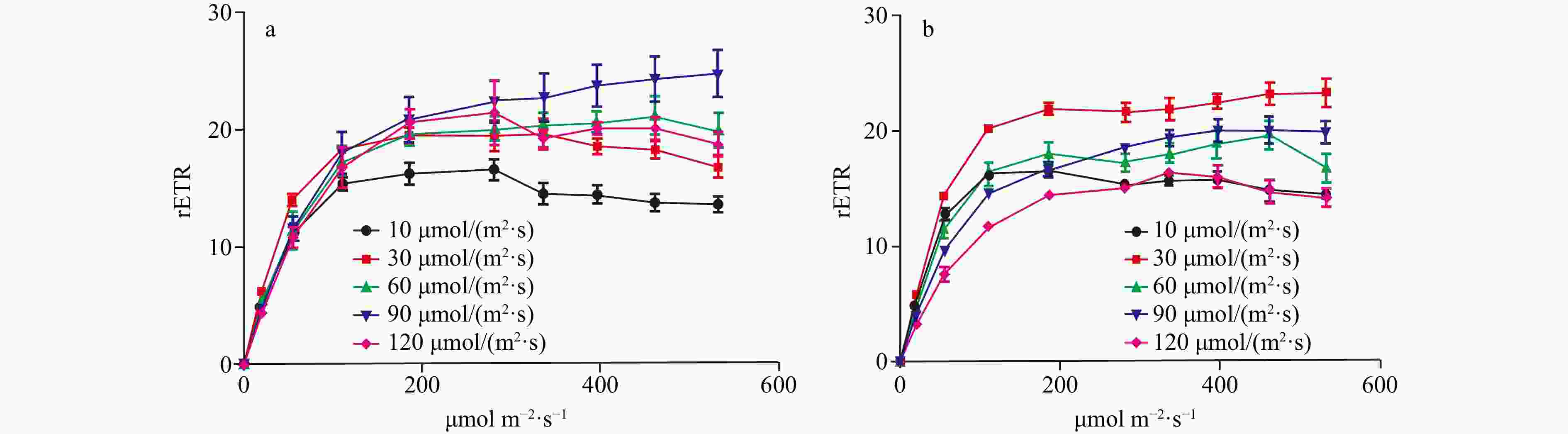
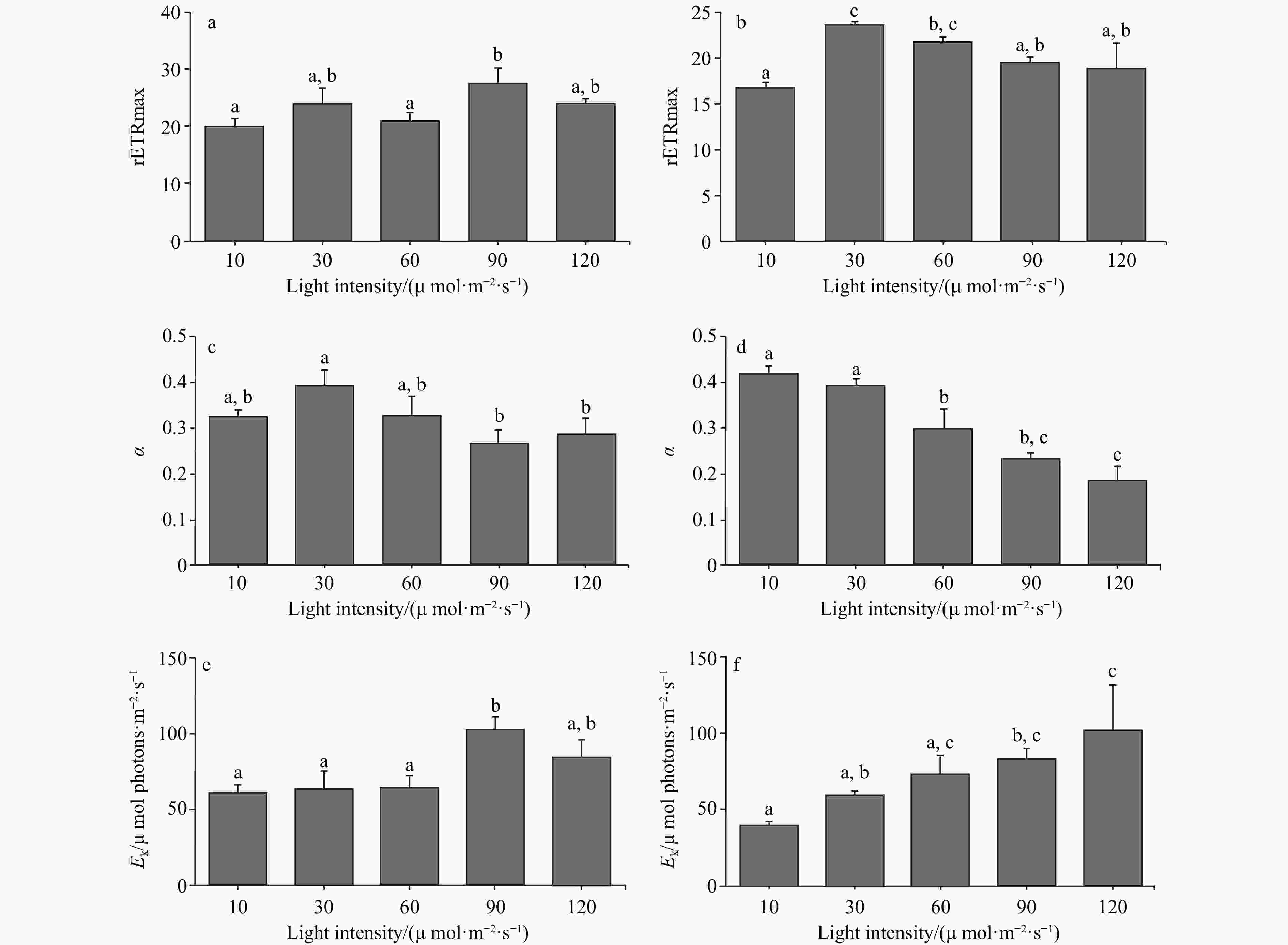
Figure 3. The rETRmax, photosynthetic rate in the light-limited region of RLC (α), and Ek of two Codium species cultured at different light intensities. The data were obtained from RLCs. a. The comparison of rETRmax of C. fragile cultured at different light intensities, b. the comparison of rETRmax of C. cylindricum cultured at different light intensities, c. the comparison of α of C. fragile cultured at different light intensities, d. the comparison of α of C. cylindricum cultured at different light intensities, e. the comparison of Ek of C. fragile cultured at different light intensities, and f. the comparison of Ek of C. cylindricum cultured at different light intensities. Different letters (a, b, and c) represent significant differences among groups.
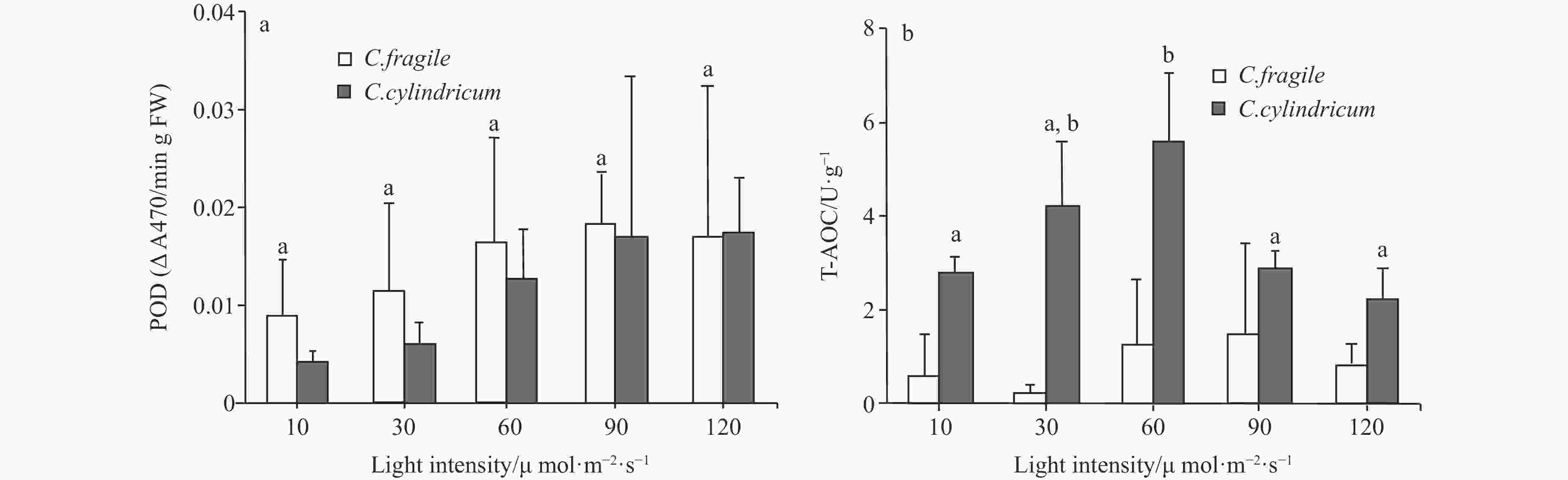
Figure 8. The comparison of POD activity (a, letters a represent no significant differences among groups (p>0.05, ANOVA, followed by Tukey's multiple comparison test)) and T-AOC (b, different letters (a and b) represent significant differences among groups (p<0.05, ANOVA, followed by Tukey's multiple comparison test)) of two Codium species under different light intensities.
Table 1. Operational procedure for the determination of T-AOC
Measurement tube Control tube Reagent 1/mL 1 1 Sample to be tested/mL a* Reagent 2/mL 2 2 Reagent 3/mL 0.5 0.5 Mix thoroughly with a vortex mixer, 37℃ water bath for 30 min Reagent 4/mL 0.2 0.2 Sample to be tested/mL a* Reagent 5/mL 0.2 0.2 Note: The reference sampling volume for 10% tissue homogenate is 0.2 mL. -
Benson E E, Rutter J C, Cobb A H. 1983. Seasonal variation in frond morphology and chloroplast physiology of the intertidal alga Codium fragile (Suringar) Hariot. New Phytologist, 95(4): 569–580, doi: 10.1111/j.1469-8137.1983.tb03522.x Bird C J, Dadswell M J, Grund D W. 1993. First record of the potential nuisance alga Codium fragile ssp. tomentosoides (Chlorophyta, Caulerpales) in Atlantic Canada. Proceedings of the Nova Scotian Institute of Science, 40(1): 11–17 Bouman H A, Platt T, Doblin M, et al. 2018. Photosynthesis–irradiance parameters of marine phytoplankton: synthesis of a global data set. Earth System Science Data, 10(1): 251–266, doi: 10.5194/essd-10-251-2018 Carlton J T, Scanlon J A. 1985. Progression and dispersal of an introduced alga: Codium fragile ssp. tomentosoides (Chlorophyta) on the Atlantic coast of North America. Botanica Marina, 28(4): 155–166, doi: 10.1515/botm.1985.28.4.155 Chapman A S. 1998. From introduced species to invader: what determines variation in the success of Codium fragile ssp. tomentosoides (Chlorophyta) in the North Atlantic Ocean? Helgolä ander Meeresuntersuchungen, 52(3-4): 277–289 Churchill A C, Moeller H W. 1972. Seasonal patterns of reproduction in New York. Populations of Codium fragile (sur. ) Hariot subsp. tomentosoides (Van Goor) Silva. Journal of Phycology, 8(2): 147–152, doi: 10.1111/j.0022-3646.1972.00147.x Ciompi S, Gentili E, Guidi L, et al. 1996. The effect of nitrogen deficiency on leaf gas exchange and chlorophyll fluorescence parameters in sunflower. Plant Science, 118(2): 177–184, doi: 10.1016/0168-9452(96)04442-1 Ding Lanping, Wang Xulei, Huang Bingxin, et al. 2022. The environmental adaptability and reproductive properties of invasive green alga Codium fragile from the Nan'ao Island, South China Sea. Acta Oceanologica Sinica, 41(3): 70–75, doi: 10.1007/s13131-021-1928-6 Dromgoole F I. 1975. Occurrence of Codium fragile subspecies tomentosoides in New Zealand waters. New Zealand Journal of Marine and Freshwater Research, 9(3): 257–264, doi: 10.1080/00288330.1975.9515564 Drouin A, McKindsey C W, Johnson L E. 2012. Detecting the impacts of notorious invaders: experiments versus observations in the invasion of eelgrass meadows by the green seaweed Codium fragile. Oecologia, 168(2): 491–502, doi: 10.1007/s00442-011-2086-x Du Xiumin, Yin Wenxuan, Zhao Yanxiu, et al. 2001. The production and scavenging of reactive oxygen species in plants. Chinese Journal of Biotechnology (in Chinese), 17(2): 121–125 Gao Guang, Liu Yameng, Li Xinshu, et al. 2016. An ocean acidification acclimatised green tide alga is robust to changes of seawater carbon chemistry but vulnerable to light stress. PLoS One, 11(12): e0169040, doi: 10.1371/journal.pone.0169040 Gao Guang, Xu Zhiguang, Shi Qi, et al. 2018. Increased CO2 exacerbates the stress of ultraviolet radiation on photosystem II function in the diatom Thalassiosira weissflogii. Environmental and Experimental Botany, 156: 96–105, doi: 10.1016/j.envexpbot.2018.08.031 Goss R, Böhme K, Wilhelm C. 1998. The xanthophyll cycle of Mantoniella squamata converts violaxanthin into antheraxanthin but not to zeaxanthin: consequences for the mechanism of enhanced non-photochemical energy dissipation. Planta, 205(4): 613–621, doi: 10.1007/s004250050364 Guidi L, Degl’Innocenti E. 2012. Chlorophyll a fluorescence in abiotic stress. In: Venkateswarlu B, Shanker A K, Shanker C, et al. , eds. Crop Stress and Its Management: Perspectives and Strategies. Dordrecht: Springer: 359–398 Hanisak M D. 1979. Growth patterns of Codium fragile ssp. tomentosoides in response to temperature, irradiance, salinity, and nitrogen source. Marine Biology, 50(4): 319–332, doi: 10.1007/BF00387009 Hanisak M D, Harlin M M. 1978. Uptake of inorganic nitrogen by Codium fragile subsp. tomentosoides (Chlorophyta). Journal of Phycology, 14(4): 450–454, doi: 10.1111/j.1529-8817.1978.tb02467.x Hui Hongxia, Xu Xing, Li Shouming. 2004. Possible mechanism of inhibition on photosynthesis of Lycium barbarum under salt stress. Chinese Journal of Ecology (in Chinese), 23(1): 5–9 Israel A, Einav R, Silva P C, et al. 2010. First report of the seaweed Codium parvulum (Chlorophyta) in Mediterranean waters: recent blooms on the northern shores of Israel. Phycologia, 49(2): 107–112, doi: 10.2216/PH09-28.1 Lapointe B E, Barile P J, Littler M M, et al. 2005. Macroalgal blooms on southeast Florida coral reefs: I. Nutrient stoichiometry of the invasive green alga Codium isthmocladum in the wider Caribbean indicates nutrient enrichment. Harmful Algae, 4(6): 1092–1105, doi: 10.1016/j.hal.2005.06.004 Leandro A, Pereira L, Gonçalves A M M. 2020. Diverse applications of marine macroalgae. Marine Drugs, 18(1): 17 Ma Ting, Li Qiang, Wang Guoxiang, et al. 2006. Influence of suspended sands on rapid light curves of Ceratophyllum demersum in turbid solution. Journal of Wuhan Botanical Research (in Chinese), 24(6): 531–535 Magnusson M, Mata L, Wang Na, et al. 2015. Manipulating antioxidant content in macroalgae in intensive land-based cultivation systems for functional food applications. Algal Research, 8: 153–160, doi: 10.1016/j.algal.2015.02.007 Malinowski K C, Ramus J. 1973. Growth of the green alga Codium fragile in a Connecticut estuary. Journal of Phycology, 9(1): 102–110, doi: 10.1111/j.0022-3646.1973.00102.x Maoka T. 2020. Carotenoids as natural functional pigments. Journal of Natural Medicines, 74(1): 1–16, doi: 10.1007/s11418-019-01364-x Marques R, Cruz S, Calado R, et al. 2021. Effects of photoperiod and light spectra on growth and pigment composition of the green macroalga Codium tomentosum. Journal of Applied Phycology, 33(1): 471–480, doi: 10.1007/s10811-020-02289-9 Marques R, Moreira A, Cruz S, et al. 2022. Controlling light to optimize growth and added value of the green macroalga Codium tomentosum. Frontiers in Marine Science, 9: 906332, doi: 10.3389/fmars.2022.906332 Matsubara K, Matsuura Y, Bacic A, et al. 2001. Anticoagulant properties of a sulfated galactan preparation from a marine green alga, Codium cylindricum. International Journal of Biological Macromolecules, 28(5): 395–399, doi: 10.1016/S0141-8130(01)00137-4 Maxwell K, Johnson G N. 2000. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany, 51(345): 659–668, doi: 10.1093/jexbot/51.345.659 Neill P E, Alcalde O, Faugeron S, et al. 2006. Invasion of Codium fragile ssp. tomentosoides in northern Chile: a new threat for Gracilaria farming. Aquaculture, 259(1-4): 202–210, doi: 10.1016/j.aquaculture.2006.05.009 Parsons T R, Strickland J. 1963. Discussion of spectrophotometric determination of marine-plant pigments, with revised equations for ascertaining chlorophylls and carotenoids. Journal of Marine Research, 21(3): 155–163 Pérez-Cirera J L, Cremades J, Bárbara I. 1989. Systematic and synecologic comments about some new seaweed records for Galicia or for the Atlantic coasts of the Iberian Peninsula. Anales del Jardín Botánico de Madrid, 46(1): 35–45 Platt T, Gallegos C L, Harrison W G. 1980. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. Journal of Marine Research, 38(4): 687–701 Porra R J. 2002. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynthesis Research, 73(1): 149–156 Ruban A V, Young A J, Pascal A A, et al. 1994. The effects of illumination on the xanthophyll composition of the photosystem II light-harvesting complexes of spinach thylakoid membranes. Plant Physiology, 104(1): 227–234, doi: 10.1104/pp.104.1.227 Schreiber U. 2004. Pulse-Amplitude-Modulation (PAM) fluorometry and saturation pulse method: an overview. In: Papageorgiou G C, Govindjee, eds. Chlorophyll A Fluorescence: A Signature of Photosynthesis. Dordrecht: Springer: 279–319 Silva P C. 1955. The dichotomous species of Codium in Britain. Journal of the Marine Biological Association of the United Kingdom, 34(3): 565–577, doi: 10.1017/S0025315400008821 Trowbridge C D. 1995. Establishment of the green alga Codium fragile ssp. tomentosoides on New Zealand rocky shores: current distribution and invertebrate grazers. Journal of Ecology, 83(6): 949–965, doi: 10.2307/2261177 Van Breusegem F, Vranová E, Dat J F, et al. 2001. The role of active oxygen species in plant signal transduction. Plant Science, 161(3): 405–414, doi: 10.1016/S0168-9452(01)00452-6 Velikova V, Yordanov I, Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Science, 151(1): 59–66, doi: 10.1016/S0168-9452(99)00197-1 Vitousek P M, Mooney H A, Lubchenco J, et al. 1957. Human domination of Earth's ecosystems. Science, 277(5325): 494–499 Wang Hui-Min David, Chen Ching-Chun, Huynh P, et al. 2015. Exploring the potential of using algae in cosmetics. Bioresource Technology, 184: 355–362, doi: 10.1016/j.biortech.2014.12.001 Wei Liyuan, Zheng Yi. 2021. Effects of temperature and salinity on growth and nutrient absorption of Codium fragile Hariot. Current Biotechnology (in Chinese), 11(2): 190–195 Williams S L. 2007. Introduced species in seagrass ecosystems: status and concerns. Journal of Experimental Marine Biology and Ecology, 350(1-2): 89–110, doi: 10.1016/j.jembe.2007.05.032 Xu Daquan, Zhang Yuzhong, Zhang Rongxian. 1992. Photoinhibition of photosynthesis in plants. Plant Physiology Communications (in Chinese), 28(4): 237–243 Yan Shanglong, Pan Chuang, Yang Xianqing, et al. 2021. Degradation of Codium cylindricum polysaccharides by H2O2-Vc-ultrasonic and H2O2-Fe2+-ultrasonic treatment: structural characterization and antioxidant activity. International Journal of Biological Macromolecules, 182: 129–135, doi: 10.1016/j.ijbiomac.2021.03.193 Yang M H, Blunden G, Huang F L, et al. 1997. Growth of a dissociated, filamentous stage of Codium species in laboratory culture. Journal of Applied Phycology, 9(1): 1–3, doi: 10.1023/A:1007996207924 Yang Xiaoqing, Zhang Suiqi, Liang Zongsuo, et al. 2004. Effects of water stress on chlorophyll fluorescence parameters of different drought resistance winter wheat cultivars seedlings. Acta Botanica Boreali-Occidentalia Sinica (in Chinese), 24(5): 812–816 Ye Jincong, Ning Yue, Zeng Zhinan, et al. 2010. A study of both temperature and underwater illuminance effect on the growth of green alga, Codium fragile (Suringar) Hariot. Journal of Fujian Fisheries (in Chinese), (4): 25–28 Yin Shuaiwen, He Xumei, Lang Fengxiang. 2007. Study on bacteriostatic activity of Codium fragile. Journal of Jinggangshan University (Natural Sciences) (in Chinese), 28(8): 45–46 Zubia M, Robledo D, Freile-Pelegrin Y. 2007. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. Journal of Applied Phycology, 19(5): 449–458, doi: 10.1007/s10811-006-9152-5 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 16
- HTML全文浏览量: 6
- 被引次数: 0



 下载:
下载: